UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
Filed by the Registrant x Filed by a party other than the Registrant ☐
Check the appropriate box:
| | | | | |
☐ | Preliminary Proxy Statement |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| x | Definitive Proxy Statement |
☐ | Definitive Additional Materials |
☐ | Soliciting Material Under §240.14a-12 |
ARCUTIS BIOTHERAPEUTICS, INC.
(Name of Registrant as Specified In Itsin its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| | | | | | | | |
| x | No fee required. |
☐ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | |
| (1) | Title of each class of securities to which transaction applies: |
| | |
| (2) | Aggregate number of securities to which transaction applies: |
| | |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | |
| (4) | Proposed maximum aggregate value of transaction: |
| | |
| (5) | Total fee paid: |
| | |
| ☐ | Fee paid previously with preliminary materials. |
| | |
| ☐ | Check box if any part of the fee is offset as providedFee computed on table in exhibit required by Item 25(b) per Exchange Act Rule 0-11(a)(2)Rules 14a-6(i)(1) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount previously paid: |
| | |
| (2) | Form, Schedule or Registration Statement No.: |
| | |
| (3) | Filing party: |
| | |
| (4) | Date Filed:0-11. |
| | |
a
ARCUTIS BIOTHERAPEUTICS, INC.
3027 Townsgate Road, Suite 300
Westlake Village, CA 91361
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON JUNE 9, 2021May 31, 2023
To the Stockholders of Arcutis Biotherapeutics, Inc.:
NOTICE IS HEREBY GIVEN that the Annual Meeting of Stockholders (the “Annual Meeting”) of Arcutis Biotherapeutics, Inc., a Delaware corporation (the “Company”), will be held virtually on June 9, 2021,May 31, 2023, at 9:008:30 a.m. local time. Stockholders can attend the meeting via the internet at www.virtualshareholdermeeting.com/ARQT2021ARQT2023 by using the 16-digit control number that appears on the accompanying Proxy Card (printed in the box and marked by the arrow) and the instructions that accompanied these proxy materials.
The Annual Meeting will be held for the following purposes:
1.To elect three Class IIII directors to hold office until the 20242026 annual meeting of stockholders or until their successors are elected;
2.To ratify the selection by the Audit Committee of the Company’s Board of Directors, of Ernst & Young LLP as the independent registered public accounting firm of the Company for its fiscal year ending December 31, 2021;2023;
3.To approve, on a non-binding advisory basis, the compensation of the Company’s named executive officers; and
3.4.To transact such other business as may properly come before the Annual Meeting or any adjournment or postponement thereof.
The foregoing items of business are more fully described in the Proxy Statement accompanying this Notice of Annual Meeting of Stockholders. Only stockholders who owned common stock of the Company at the close of business on April 13, 20213, 2023 (the “Record Date”), can vote at this meeting or any adjournments that take place.
The Board of Directors recommends that you vote FORvote:
•“FOR” the election of the director nominees named in Proposal No. 1 of the Proxy Statement; and FOR
•“FOR” the ratification of the appointmentselection of Ernst & Young LLP as the independent registered public accounting firm as described in Proposal No. 2 of the Proxy Statement.Company for its fiscal year ending December 31, 2023; and
•“FOR” the approval, on a non-binding advisory basis, of the compensation of the Company’s named executive officers.
YOUR VOTE IS IMPORTANT. WHETHER OR NOT YOU PLAN TO ATTEND THE VIRTUAL ANNUAL MEETING, WE ENCOURAGE YOU TO READ THE ACCOMPANYING PROXY STATEMENT AND OUR ANNUAL REPORT ON FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2020,2022, AND SUBMIT YOUR PROXY AS SOON AS POSSIBLE USING ONE OF THE THREE CONVENIENT VOTING METHODS DESCRIBED IN THE “INFORMATION ABOUT THE PROXY PROCESS AND VOTING” SECTION IN THE PROXY STATEMENT. IF YOU RECEIVE MORE THAN ONE SET OF PROXY MATERIALS OR NOTICE OF INTERNET AVAILABILITY BECAUSE YOUR SHARES ARE REGISTERED IN DIFFERENT NAMES OR ADDRESSES, EACH PROXY SHOULD BE SIGNED AND SUBMITTED TO ENSURE THAT ALL OF YOUR SHARES WILL BE VOTED.
| | | | | |
| By Order of the Board of Directors |
| |
| /s/ Todd Franklin Watanabe |
| Todd Franklin Watanabe |
| President, Chief Executive Officer and Director |
| Westlake Village, California | |
April 27, 202118, 2023 | |
TABLE OF CONTENTS
a
ARCUTIS BIOTHERAPEUTICS, INC.
3027 Townsgate Road, Suite 300
Westlake Village, CA 91361
PROXY STATEMENT
FOR THE 20212023 ANNUAL MEETING OF STOCKHOLDERS
JUNE 9, 2021May 31, 2023
We have sent you this Proxy Statement and the enclosed Proxy Card because the Board of Directors (the “Board”) of Arcutis Biotherapeutics, Inc. (referred to herein as the “Company”, “Arcutis”, “we”, “us” or “our”), is soliciting your proxy to vote at our 20212023 Annual Meeting of Stockholders (the “Annual Meeting”) to be held on Wednesday, June 9, 2021,May 31, 2023, at 9:008:30 a.m. local time, virtually at www.virtualshareholdermeeting.com/ARQT2021.ARQT2023. There will be no physical meeting location. The meeting will only be conducted via an audio webcast.
•This Proxy Statement summarizes information about the proposals to be considered at the Annual Meeting and other information you may find useful in determining how to vote.
•The Proxy Card is the means by which you actually authorize another person to vote your shares in accordance with your instructions.
In addition to solicitations by mail, our directors, officers and regular employees, without additional remuneration, may solicit proxies by telephone, e-mail and personal interviews. We may retain outside consultants to solicit proxies on our behalf as well. All costs of solicitation of proxies will be borne by us. Brokers, custodians and fiduciaries will be requested to forward proxy soliciting material to the owners of stock held in their names, and we will reimburse them for their reasonable out-of-pocket expenses incurred in connection with the distribution of proxy materials.
Pursuant to the rules adopted by the Securities and Exchange Commission (the “SEC”), we have elected to provide access to our Annual Meeting materials, which include this Proxy Statement and our Annual Report on Form 10-K for the year ended December 31, 20202022 (the “Form 10-K”), over the internet in lieu of mailing printed copies. We will begin mailing the Notice of Internet Availability to our stockholders of record as of April 13, 20213, 2023 (the “Record Date”), for the first time on or about April 27, 2021.18, 2023. The Notice of Internet Availability will contain instructions on how to access and review the Annual Meeting materials, and will also contain instructions on how to request a printed copy of the Annual Meeting materials. In addition, we have provided brokers, dealers, banks, voting trustees and their nominees, at our expense, with additional copies of our proxy materials and the Form 10-K so that our record holders can supply these materials to the beneficial owners of shares of our common stock as of the Record Date. The Form 10-K is also available in the “Financials” section of our website at https://investors.arcutis.com/investor-relations.
The only outstanding voting securities of Arcutis are shares of common stock, $0.0001 par value per share (the “common stock”), of which there were 50,149,74461,360,936 shares outstanding as of the Record Date (excluding any treasury shares). The holders of a majority in voting power of the shares of common stock issued and outstanding and entitled to vote, present in person or represented by proxy, are required to hold the Annual Meeting.
INFORMATION ABOUT THE PROXY PROCESS AND VOTING
Why am I receiving these materials?
We have made this Proxy Statement and Proxy Card available to you on the internet or, upon your request, have delivered printed proxy materials to you, because the Board is soliciting your proxy to vote at the Annual Meeting, including at any adjournments or postponements of the Annual Meeting. You are invited to attend the Annual Meeting via internet to vote on the proposals described in this Proxy Statement. However, you do not need to attend the Annual Meeting to vote your shares. Instead, you may simply complete, sign and return the Proxy Card, or follow the instructions below to submit your proxy over the telephone or on the internet.
This Proxy Statement, the Notice of Internet Availability, the Notice of Annual Meeting and accompanying Proxy Card will be first made available for access by our stockholders on or about April 27, 2021,18, 2023, to all stockholders of record entitled to vote at the Annual Meeting.
Who can vote at the Annual Meeting?
Only stockholdersStockholders of record at the close of business on the Record Date will be entitled to notice of and to vote at the Annual Meeting. At the close of business on the Record Date, there were 50,149,74461,360,936 shares of common stock issued and outstanding and entitled to vote. Each share of our common stock is entitled to one vote on any matter presented to stockholders at the Annual Meeting. You will need to obtain your own internet access if you choose to attend the Annual Meeting online and/or vote over the internet.
Stockholder of Record: Shares Registered in Your Name
If, on the Record Date, your shares were registered directly in your name with the transfer agent for our common stock, Equiniti Trust Company, then you are a stockholder of record. As a stockholder of record, you may vote at the virtual Annual Meeting or vote by proxy. Whether or not you plan to attend the Annual Meeting, we encourage you to fill out and return the Proxy Card or vote by proxy over the telephone or on the internet as instructed below (see “How do I vote?”) to ensure your vote is counted.
Beneficial Owner: Shares Registered in the Name of a Broker, Bank or Other Agent
If, on the Record Date, your shares were held in an account at a brokerage firm, bank, dealer or other similar organization, then you are the beneficial owner of shares held in “street name” and these proxy materials are being forwarded to you by that organization. The organization holding your account is considered the stockholder of record for purposes of voting at the Annual Meeting. As a beneficial owner, you have the right to direct your broker or other agent on how to vote the shares in your account. You are also invited to attend the virtual Annual Meeting. However, since you are not the stockholder of record, you may not vote your shares at the virtual Annual Meeting unless you request and obtain a valid Proxy Card from your broker or other agent.
What am I being asked to vote on?
YouThere are being asked to vote on two proposals:four matters scheduled for a vote:
•Proposal No. 1 - the election of three Class IIII directors to hold office until our 20242026 annual meeting of stockholders; and
•Proposal No. 2 - the ratification of the selection by the Audit Committee of our Board, of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2021.2023; and
•Proposal No. 3 - the approval, on a non-binding advisory basis, of the compensation of our named executive officers.
In addition, you are entitled to vote on any other matters that are properly brought before the Annual Meeting. The Board knows of no other matters that will be presented for consideration at the Annual Meeting.
What is the Board’s voting recommendation?
The Board recommends that you vote your shares:
4•“FOR” the election of the director nominees;
•“FOR” the ratification of the selection of Ernst & Young LLP as the independent registered public accounting firm for the Company’s fiscal year ending December 31, 2023; and
•“FOR” the approval, on a non-binding advisory basis, of the compensation of the named executive officers.How do I vote?
•For Proposal No. 1, you may either vote “For” all the nominees to the Board or you may “Withhold” your vote for any nominee you specify.
•For Proposal Nos. 2 and 3, you may either vote “For” or “Against” or abstain from voting.
Please note that by casting your vote by proxy you are authorizing the individuals listed on the Proxy Card to vote your shares in accordance with your instructions and in their discretion with respect to any other matter that properly comes before the Annual Meeting or any adjournments or postponements thereof.
The procedures for voting depend on whether your shares are as follows:registered in your name or are held by a bank, broker or other agent:
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record, you may vote at the virtual Annual Meeting. Alternatively, you may vote by proxy, by using the accompanying Proxy Card, over the internet or by telephone. Whether or not you plan to attend the Annual Meeting, we encourage you to vote by proxy to ensure your vote is counted. Even if you have submitted a proxy before the Annual Meeting, you may still attend the virtual Annual Meeting and vote by following the instructions described below. In such case, your previously submitted proxy will be disregarded.
•At the Annual Meeting - To vote by attending the virtual Annual Meeting, vote your shares at www.virtualshareholdermeeting.com/ARQT2021ARQT2023 during the Annual Meeting. You will need the 16-digit control number which appears on the accompanying Proxy Card (printed in the box and marked by the arrow) and the instructions that accompanied these proxy materials. For additional details on the virtual meeting, please see page 9[9] of this Proxy Statement.
•By Mail - To vote using the Proxy Card, simply complete, sign and date the accompanying Proxy Card and return it promptly in the envelope provided. If you return your signed Proxy Card to us before the Annual Meeting, we will vote your shares in accordance with the Proxy Card.
•Via the Internet - To vote by proxy over the internet, follow the instructions provided on the Notice of Internet Availability.
•By Telephone - To vote by telephone, you may vote by proxy by calling the toll free number found on the Notice of Internet Availability.
Beneficial Owner: Shares Registered in the Name of Broker, Bank or Other Agent
If you are a beneficial owner of shares registered in the name of your broker, bank or other agent, you should have receivedwill receive a voting instruction card and voting instructions with these proxy materials from that organization rather than from us. Simply complete and mail the voting instruction card to ensure that your vote is counted. To vote at the virtual Annual Meeting, you must obtain a valid proxy from your broker, bank or other agent. Follow the instructions from your broker, bank or other agent included with these proxy materials, or contact your broker, bank or other agent to request a proxy form.
We provide internet proxy voting to allow you to vote your shares online, with procedures designed to ensure the authenticity and correctness of your proxy vote instructions. However, please be aware that you must bear any costs associated with your internet access, such as usage charges from internet access providers and telephone companies.
Who counts the votes?
Broadridge Financial Solutions, Inc. (“Broadridge”), has been engaged as our independent agent to tabulate stockholder votes or and a representative of Broadridge will act as inspector of election (“Inspector of Election. If you are a stockholder of record, your executed Proxy Card is returned directly to Broadridge for tabulation. As noted above, if you hold your shares through a broker, your broker returns one Proxy Card to Broadridge on behalf of all its clients.
How are votes counted?
Votes will be counted by the Inspector of Election appointed for the Annual Meeting, who will separately count “For” votes for all proposals, and, with respect to Proposal 2, “Against” votes, abstentions and broker non-votes. In addition, with respect to Proposal 1, the election of directors, the Inspector of Election will count the number of “Withheld” votes and broker non-votes received. If your shares are held by your broker as your nominee (that is, in “street name”Election”), you will need to obtain a proxy form from the institution that holds your shares and follow the instructions included on that form regarding how to instruct your broker to vote your shares. If you do not give instructions to your broker, your broker can vote your shares with respect to “routine” items, but not with respect to “non-routine” items. See below for more information regarding: “What are “broker non-votes”?” and “Which ballot measures are considered “routine” or “non-routine”?”.
What are “broker non-votes”?
Broker non-votes occurA “broker non-vote” occurs when a beneficial owner of shares held in “street name” does not give instructions to the broker, bank or nomineeother agent holding the shares as to how to vote on matters deemed “non-routine.”“non-routine” proposals. Generally, if shares are held in street name, the beneficial owner of the shares is entitled to give voting instructions to the broker, bank or nomineeother agent holding the shares. If the beneficial owner does not provide voting instructions, the broker, bank or nomineeother agent can still vote the shares with respect to matters that are considered to be “routine,”“routine” under the applicable rules but notcannot vote with respect to “non-routine” matters. InOn non-routine matters, any “uninstructed shares” may not be voted by the event that a broker, bank custodian, nominee or other record holderagent and are considered to be “broker non-votes.”
Broker non-votes and abstentions are counted for the purpose of common stock indicatesdetermining whether a quorum is present at the Annual Meeting. Only affirmative and negative votes are counted for purposes of determining the votes received in connection with each proposal. Broker non-votes and abstentions will have no effect on determining whether the affirmative vote constitutes a majority of the shares present or represented by proxy that it does not have discretionary authority to vote certain shares on a particular proposal, then those shares will be treated as broker non-votes with respect to that proposal.and voting at the Annual Meeting. Accordingly, if you own shares through a nominee, such as a broker, bank or bank,other agent, please be sure to instruct your nominee how to vote to ensure that your vote is counted on each of the proposals.
Which ballot measuresproposals are considered “routine” or “non-routine?”routine and which are non-routine?
The following proposal is considered a routine matter:
•Proposal No. 2 (the ratification of the appointmentselection of Ernst & Young LLP as ourthe independent registered public accounting firm for the Company’s fiscal year ending December 31, 2021 (Proposal 2) is considered routine under applicable rules. 2023).
A broker, bank or other nominee mayagent generally vote on routine matters,has discretionary voting power with respect to such proposal and therefore no broker non-votes are expected to exist in connection with Proposal No. 2.
The following proposals are considered non-routine matters:
•Proposal No. 1 (the election of directors (Proposal 1) is considered non-routine under applicable rules. director nominees); and
•Proposal No. 3 (the non-binding advisory vote to approve the compensation of the named executive officers).
A broker, bank or other nomineeagent generally cannot vote with respect to such proposals without voting instructions on non-routine matters,from the respective beneficial owner and therefore there may be broker non-votes on such proposals. If you own shares through a nominee, such as a broker, bank or other agent, and do not
instruct your nominee how to vote your shares for these proposals, the nominee will inform the Inspector of Election that it does not have the authority to vote on the matter with respect to your shares, which is referred to above as a “broker non-vote.” Therefore, broker non-votes may exist in connection with Proposal 1.No. 1 and Proposal No. 3.
How many votes are needed to approve theeach proposal?
With respect to Proposal No. 1 (the election of director nominees), the three nominees who receive the most “For” votes cast will be elected as Class III directors to our Board. Abstentions and broker non-votes are not considered votes cast and will not be counted in determining the outcome of the election of directors, the threedirector nominees receiving the highest number of “For” votes will be elected.
With respect to Proposal No. 2 (the ratification of the selection of Ernst & Young LLP as the independent registered public accounting firm for the Company’s fiscal year ending December 31, 2023), the affirmative vote of the majority of total votes cast affirmatively or negatively (excluding abstentions and broker non-votes) is required for approval. ThisAbstentions and broker non-votes are not considered votes cast, however, Proposal No. 2 is a routine proposalconsidered to be “routine” under applicable laws and thereforethus we do not expect any broker non-votes.
How many votes do I have?
On each matterWith respect to be voted upon, you have oneProposal No. 3 (the non-binding advisory vote for each share of common stock you own asto approve the compensation of the Record Date.
named executive officers), the affirmative vote of a majority of total votes cast affirmatively or negatively is required to determine approval on an advisory basis. Abstentions and broker non-votes are not considered votes cast and will not be counted in determining the outcome of the advisory vote. This vote is advisory and not binding on us, our Board, or our Compensation Committee.What if I return a Proxy Card but do not make specific choices?
If we receive a signed and dated Proxy Card and the Proxy Card does not specify how your shares are to be voted, your shares will be voted in accordance with the recommendations of the Board. The Board’s recommendations are set forth above, as follows:
•“For”well as with the electiondescription of each of the three nominees for director; and
•“For” the ratification of the appointment of Ernst & Young LLP, as our independent registered public accounting firm for the fiscal year ending December 31, 2021.
If any other matter is properly presented at the Annual Meeting, your proxy (one of the individuals named on yourproposal in this Proxy Card) will vote your shares in his or her discretion.Statement.
Who is paying for this proxy solicitation?
We have retained Morrow Sodali LLC, 333 Ludlow Street, Fifth Floor, South Tower, Stamford, CT 06902, to assist in the solicitation of proxies for a fee of approximately $7,500, plus distribution costs and other costs and expenses. We will pay for the entire cost of soliciting proxies. In addition to these mailed proxy materials, our directors, officers and employees may also solicit proxies in person, by telephone or by other means of communication. Directors, officers and employees will not be paid any additional compensation for soliciting proxies. We may also reimburse brokerage firms, banks and other agents for the cost of forwarding proxy materials to beneficial owners.
What does it mean if I receive more than one set of materials?
If you receive more than one set of materials, your shares are registered in more than one name or are registered in different accounts. In order to vote all the shares you own, you must either sign and return all of the Proxy Cards or follow the instructions for any alternative voting procedure on each of the Proxy Cards.
Can I change my vote after submitting my proxy?
Yes. You can revoke your proxy at any time before the final vote at the Annual Meeting. If you are the record holder of your shares, you may revoke your proxy in any one of three ways:the following ways on or before the close of voting for the Annual Meeting:
•You may submit another properly completed proxy with a later date.
•You may grant a subsequent timely proxy by telephone or through the internet.
•You may send a timely written notice that you are revoking your proxy to our Corporate Secretary at 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361.
•You may attend the virtual Annual Meeting and vote at the meeting by following the instructions described above. Simply attending the Annual Meeting will not, by itself, revoke your proxy.
Your most current proxy card or telephone or internet proxy is the one that is counted, so long as it is provided within the applicable deadline. If your shares are held by your broker, bankbanker or other agent, you should follow the instructions provided by them.your broker, bank or other agent to change your vote or revoke your proxy.
How do I attend the virtual Annual Meeting?
The live audio webcast of the Annual Meeting. Any stockholder can attend the Annual Meeting will begin promptlylive online at 9:00 a.m. local time.www.virtualshareholdermeeting.com/ARQT2023. Online access to the audio webcast will open approximately 15 minutes prior to the start of the Annual Meeting to allow time for our stockholders to log in and test their devices’ audio system. We encourage our stockholders to access the meeting in advance of the designated start time.
ToIf you were a stockholder as of the Record Date, or you hold a valid proxy for the Annual Meeting, you can vote at the Annual Meeting. A summary of the information you need to attend the Annual Meeting stockholdersonline is provided below:
•Instructions on how to attend and participate via the internet, including how to demonstrate proof of stock ownership, are posted at www.virtualshareholdermeeting.com/ARQT2023.
•Assistance with questions regarding how to attend and participate via the internet will be provided at www.virtualshareholdermeeting.com/ARQT2023 on the day of the Annual Meeting.
•Webcast starts at 8:30 a.m. Pacific time.
•You will need to log-in to www.virtualshareholdermeeting.com/ARQT2021 using theyour 16-digit control number on the proxy card or voting instruction form.
Can I submit questions prior to or at the virtual Annual Meeting?
Stockholders may submit questions and vote on the day of, or during, the Annual Meeting on www.virtualshareholdermeeting.com/ARQT2021.ARQT2023. To demonstrate proof of stock ownership, you will need to enter the 16-digit control number received with your proxy card or voting instruction form to submit questions and vote at our Annual Meeting. We intend to answer questions submitted during the meeting that are pertinent to the Company and the items being brought before stockholder vote at the Annual Meeting, as time permits, and in accordance with the Rules of Conduct for the Annual Meeting. Questions and answers will be grouped by topic and substantially similar questions will be answered only once. To promote fairness, efficiently use the Company’s resources and ensure all stockholder questions are able to be addressed, we will respond to no more than three questions from a single stockholder.
Is technical assistance provided before and during the virtual Annual Meeting?
If you encounter any difficulties accessing the virtual meeting during the check-in time or meeting time, or you have any questions regarding how to use the virtual meeting platform, please call the technical support number that will be posted on the virtual shareholder meeting log-in page.
When are stockholder proposals due for next year’s Annual Meeting?
Stockholders who intend to have a proposal considered for inclusion in our proxy materials for presentation at our 20222024 Annual Meeting of Stockholders pursuant to Rule 14a-8 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) must submit the proposal to our Corporate Secretary at 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361 no later than December 18, 2021.20, 2023.
Stockholders intending to present a proposal at the 20222024 Annual Meeting of Stockholders, but not to include the proposal in our proxy statement, or to nominate a person for election as a director, must comply with the requirements in our bylaws. Our bylaws require, among other things, that our Corporate Secretary receive written notice from the stockholder of record of their intent to present such proposal or nomination not less than 90 days nor more than 120 days prior to the first anniversary of the preceding year’s annual meeting. Therefore, we must receive notice of such a proposal or nomination for the 20222024 Annual Meeting of Stockholders no earlier than the close of business on February 10, 20221, 2024 and no later than the close of business on March 11, 2022.2, 2024. The notice must contain the information required by the bylaws, a copy of which is available upon request to our Corporate Secretary. In the event that the date of the 20222024 Annual Meeting of Stockholders is more than 30 days before or more than 30 days after June 9, 2022,May 31, 2024, then our Corporate Secretary must receive such written notice not earlier than the close of business on the 120th day prior to the 20222024 Annual Meeting and not later than the close of business on the 90th day prior to the 20222024 Annual Meeting or, if later, the 10th day following the day on which public disclosure of the date of such annual meeting is first made by us. You are advised to review our bylaws, which contain additional requirements about advance notice of stockholder proposals and director nominations. In addition to satisfying the foregoing requirements under the Company’s Bylaws, to comply with the universal proxy rules, any notice of director nomination submitted to the Company must include the additional information required by Rule 14a-19 under the Exchange Act no later than April 1, 2024.
We intend to file our Proxy Statement and WHITE proxy card with the SEC in connection with our solicitation of proxy for our 2023 Annual Stockholders Meeting. Stockholders may obtain our Proxy Statement (and any amendments or supplements thereto) and other documents as and when filed by the Company with the SEC without charge from the Company’s website at www.sec.gov.
What is the quorum requirement?
A quorum of stockholders is necessary to hold a valid meeting. A quorum will be present if the holders of a majority in voting power of the shares of common stock issued and outstanding and entitled to vote are present in person, or by remote communication, if applicable, or represented by proxy at the Annual Meeting. Shares are considered present “in person” if voted by the holder of those shares during the Annual Meeting or by proxy. On the Record Date, there were 50,149,74461,360,936 shares outstanding and entitled to vote. Accordingly, 25,074,87330,680,469 shares must be represented by stockholders present at the Annual Meeting or by proxy to have a quorum.
Your shares will be counted toward the quorum only if you submit a valid proxy or vote at the Annual Meeting. Abstentions and broker non-votes will be counted toward the quorum requirement. If there is no quorum, either the Chair of the Annual Meeting or a majority in voting power of the stockholders entitled to vote at the Annual Meeting, present in person, or by remote communication, if applicable, or represented by proxy, may adjourn the Annual Meeting to another time or place.
How can I find out the results of the voting at the Annual Meeting?
Voting results will be announced by the filing of a Current Report on Form 8-K within four business days after the Annual Meeting. If final voting results are unavailable at that time, we will file an amended Current Report on Form 8-K within four business days of the day the final results are available.
Implications of being an “emerging growth company.”
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements. These reduced reporting requirements include reduced disclosure about our executive compensation arrangements and no non-binding advisory votes on executive compensation. We will remain an emerging growth company until the earlier of: (1) (a) December 31, 2025, (b) the last day of the fiscal year in which we have total annual gross revenue of at least $1.07 billion, or (c) the last day of the fiscal year in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
a
PROPOSAL NO. 1
ELECTION OF DIRECTORS
Board Size and Structure
Our Board is divided into three classes. Each class consists, as nearly as possible, of one-third of the total number of directors, and each class has a staggered, three-year term. Unless the Board determines that vacancies (including vacancies created by increases in the number of directors) shall be filled by the stockholders, and except as otherwise provided by law, vacancies on the Board may be filled only by the affirmative vote of a majority of the remaining directors. A director elected by the Board to fill a vacancy (including a vacancy created by an increase in the number of directors) shall serve for the remainder of the full term of the class of directors in which the vacancy occurred and until such director’s successor is elected and qualified.
The Board currently consists of 910 seated directors, divided into the three following classes:
•Class I directors: Terrie Curran, Halley Gilbert and Ricky Sun, Ph.D.Keith R. Leonard, Jr., whose current terms will expire at the Annual Meeting;
•Class II directors: Bhaskar Chaudhuri, Ph.D., Howard Welgus, M.D. and Jonathan Silverstein, whose current terms will expire at the annual meeting of stockholders to held be in 2022; and
•Class III directors: Patrick Heron, Joseph L. Turner, and Todd Franklin Watanabe, whose current terms will expire at the annual meeting of stockholders to be held in 2023.2024;
•Class II directors: Bhaskar Chaudhuri, Ph.D., Howard Welgus, M.D. and Sue-Jean Lin, whose current terms will expire at the annual meeting of stockholders to be held in 2025; and
•Class III directors: Patrick Heron, Neha Krishnamohan, Joseph L. Turner, and Todd Franklin Watanabe, whose current terms will expire at the Annual Meeting.
At each annual meeting of stockholders, the successors to directors whose terms will then expire will be elected to serve from the time of election and qualification until the third subsequent annual meeting of stockholders.
Mr. Heron, Ms. Curran, Ms. GilbertKrishnamohan and Dr. SunMr. Watanabe have been nominated to serve as Class IIII directors and have each elected to stand for reelection. Each director to be elected will hold office from the date of their election by the stockholders until the third subsequent annual meeting of stockholders or until his or her successor is elected and has been qualified, or until such director’s earlier death, resignation or removal. Mr. Turner will not stand for reelection. Mr. Turner will continue to serve as a director until the expiration of his term at the Annual Meeting. The Board expresses its gratitude to Mr. Turner for his many contributions during his service on the Board. Our Bylaws provide that the number of directors will be determined by the Board, and the number of directors is currently set at 10. The Board will reduce the number of directors to 9 following the Annual Meeting.
Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of thethese three nominees named below.nominees. In the event that any nominee should be unavailable for election as a result of an unexpected occurrence, such shares will be voted for the election of such substitute nominee as the Board may propose. Each person nominated for election has agreed to serve if elected, and management has no reason to believe that any nominee will be unable to serve. Directors are elected by a plurality of the votes cast at the meeting.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE
FOR THE ELECTION OF EACH NAMED NOMINEE.
The following table sets forth, for the Class IIII nominees (who are currently standing for re-election) and for our other current directors who will continue in office after the Annual Meeting, information with respect to their ages as of April 13, 2021,3, 2023, tenure on the Board, and position/office held within the Company:
| | | | | | | | | | | | | | | | | | | | |
| Name | | Age | | Position/Office Held With the Company | | Director Since |
| Class I Directors whose terms expire at the Annual Meeting of Stockholders | | |
| Terrie Curran (3) | | 52 | | Director | | 2020 |
| Halley Gilbert (1) | | 51 | | Director | | 2020 |
| Ricky Sun, Ph.D. (3) | | 47 | | Director | | 2018 |
| | | | | | |
| Class II Directors whose terms expire at the 2022 Annual Meeting of Stockholders | | |
| Bhaskar Chaudhuri, Ph.D. (1) (2) | | 66 | | Director | | 2016 |
| Howard G. Welgus, M.D. (3) | | 69 | | Director | | 2020 |
| Jonathan Silverstein (2) | | 53 | | Director | | 2018 |
| | | | | | |
| Class III Directors whose terms expire at the 2023 Annual Meeting of Stockholders | | |
| Patrick Heron (2) | | 50 | | Chairman and Director | | 2016 |
| Joseph L. Turner (1) | | 69 | | Director | | 2020 |
| Todd Franklin Watanabe | | 53 | | President, Chief Executive Officer and Director | | 2017 |
| | | | | | | | | | | | | | | | | | | | |
| Name | | Age | | Position/Office Held With the Company | | Director Since |
| Class I Directors whose terms expire at the 2024 Annual Meeting of Stockholders | | |
| Terrie Curran (3) | | 54 | | Director | | 2020 |
| Halley E. Gilbert (1) | | 53 | | Director | | 2020 |
| Keith R. Leonard, Jr. (2) | | 61 | | Director | | 2021 |
| | | | | | |
| Class II Directors whose terms expire at the 2025 Annual Meeting of Stockholders | | |
| Bhaskar Chaudhuri, Ph.D. (2) | | 68 | | Director | | 2016 |
| Howard G. Welgus, M.D. (3) | | 71 | | Director | | 2020 |
| Sue-Jean Lin (1) (3) | | 64 | | Director | | 2021 |
| | | | | | |
| Class III Directors whose terms expire at the Annual Meeting of Stockholders | | |
| Patrick J. Heron (2) | | 52 | | Chairman and Director | | 2016 |
| Neha Krishnamohan (1) | | 36 | | Director | | 2022 |
| Todd Franklin Watanabe | | 55 | | President, Chief Executive Officer and Director | | 2017 |
(1)Member of the Audit Committee.
(2)Member of the Compensation Committee.
(3)Member of the Nominating and Corporate Governance Committee.
Set forth below is biographical information for the nominees and each person whose term of office as a director will continue after the Annual Meeting. The following includes certain information regarding our directors’ individual experience, qualifications, attributes and skills that led the Board to conclude that they should serve as directors.
Nominees for Election to a Three-Year Term Expiring at the 2026 Annual Meeting of Stockholders
Patrick J. Heron has served as the Chair of our board of directors since December 2019, and has been a member of our board of directors since April 2016. Since September 1999, Mr. Heron has been a managing general partner with Frazier Life Sciences, where he has been active in company formations and initial investments in various biotechnology companies, including Marcadia Biotech Inc., Calixa Therapeutics, Inc. and VentiRx Pharmaceuticals, Inc. He also led Frazier’s involvement in MedPointe Inc. Prior to joining Frazier, Mr. Heron helped develop McKinsey & Company’s west coast biotechnology consulting practice. Mr. Heron currently serves on the board of directors of several private companies and the following public companies: Mirum Pharmaceuticals, Inc., and HilleVax, Inc. He previously served on the board of directors of several public companies including Vaxcyte, Inc. (2017 to 2021), Passage Bio, Inc. (2018 to 2021), Iterum Therapeutics, plc. (2014 to 2022), Imago Biosciences, Inc. (2014 to 2022). Mr. Heron received a B.A. in Political Science from the University of North Carolina at Chapel Hill and an M.B.A. from Harvard Business School. We believe that Mr. Heron is qualified to serve on our board of directors because of his investing and operations experiences in the life sciences industry.
Neha Krishnamohan has served as a member of our board of directors since September 2022. Since June 2021, Ms. Krishnamohan has served as Chief Financial Officer and Executive Vice President, Corporate Development at Kinnate Biopharma Inc., a publicly traded biopharmaceutical company. Prior to joining Kinnate, she was with Goldman Sachs where she held various roles since July 2008, most recently as Vice President in the Healthcare Investment Banking Group. During her tenure at Goldman Sachs, she advised a variety of biopharmaceutical company boards of directors and management teams on a broad range of strategic financial matters; and executing financings and leading M&A transactions. Ms. Krishnamohan holds a B.S.E. with a double major in Biomedical Engineering and Economics from Duke University. We believe that Ms. Krishnamohan is qualified to serve on our board of directors
because of her business and financial expertise, including capital markets, and her experience as a senior executive in the biopharmaceutical industry.
Todd Franklin Watanabe has served as our President and Chief Executive Officer since April 2017. Prior to joining Arcutis Biotherapeutics, he served as co-founder and Chief Operating Officer of Kanan Therapeutics, Inc., a private cardiovascular drug development company from December 2015 to February 2018, and before that, he served as Vice President of Strategy and Corporate Development at Kythera Biopharmaceuticals Inc. from October 2013 to November 2015. Mr. Watanabe was an executive at Amgen, Inc. from 2005 to 2013, where he was involved in the development of Repatha for hyperlipidemia and Aimovig for migraine, and worked on the U.S. marketing of Enbrel in both dermatology and rheumatology. Previously, he was an executive with Eli Lilly and Company, and an official in the U.S. Government. He was also a commissioned officer in the U.S. Navy Reserves for 25 years. Mr. Watanabe received his M.A. in National Security Studies, and his B.A. in International Relations, both from Georgetown University. We believe that Mr. Watanabe is qualified to serve on our board of directors because of his expertise in dermatology and experience with biotechnology companies, including working with and serving in various executive positions in life sciences companies.
Directors Continuing in Office Until the 2024 Annual Meeting of Stockholders
Terrie Curran has served as a member of our board of directors since November 2020.2020 and has served as the Chair of the Nominating and Corporate Governance Committee since 2021. Ms. Curran has served as the Chief Executive Officer and President of Phathom Pharmaceuticals, Inc., a late clinical-stage biopharmaceutical company focused on developing and commercializing new treatments for gastrointestinal diseases, since December 2019, and has served as a member of its board of directors since August 2019. Since November 2016, Ms. Curran has served as a member of the board of directors of Myovant Sciences Ltd., a clinical-stage biopharmaceutical company with a focus on treatments for women suffering from uterine fibroids, endometriosis and infertility and men suffering from prostate cancer. Ms. Curran previously served as the President, Global Inflammation and Immunology (I&I) Franchise at Celgene Corporation and a member of its Executive Committee from April 2017 until November 2019. Ms. Curran joined Celgene in 2013 as the U.S. Commercial Head of the I&I Franchise and built the capabilities and recruited the teams that executed the successful launch of OTEZLA, before becoming Head of Worldwide Markets. Prior to joining Celgene, she served as Senior Vice President and General Manager - Global Women’s Health at Merck & Co. Before joining Merck, Ms. Curran held a number of Country General Manager positions at Schering-Plough and Pharmacia across Europe and Asia Pacific. She previously served on the boardboards of Myovant Sciences Ltd. and H. Lundbeck A/S, a global pharmaceutical company. Ms. Curran holds a Graduate Diploma of Marketing and a Bachelor of Applied Science (B.A.S.) from the University of Technology, Sydney. We believe Ms. Curran is qualified to serve on our board of directors because of her manyexpertise in dermatology and years of experience in the pharmaceutical industry, including positions in senior executive roles at major pharmaceutical companies.
Halley E. Gilbert has served as a member of our board of directors since May 2020. SinceFrom August 2021 through April 2022, Ms. Gilbert served as Chief Legal Officer of NeoGenomics Laboratories, a cancer diagnostics company. From July 2020 to August 2021, Ms. Gilbert has served as Chief Operating Officer of Invivyd, Inc. (formerly, Adagio Therapeutics, Inc.Therapeutics), a clinical stage company developing antibodies that seekfor infectious diseases. Prior to neutralize SARS-Cov-2, and additional potential emergent coronaviruses. Since April 2020,Invivyd, Ms. Gilbert hasheld various roles at Ironwood Pharmaceuticals from February 2008 through February 2020, including Senior Vice President for Corporate Development, Chief Administrative Officer, and Chief Legal Officer. Prior to joining Ironwood Pharmaceuticals, Ms. Gilbert was Vice President, Deputy General Counsel at Cubist Pharmaceuticals, Inc. and previously served as a corporate counsel at Genzyme Corp., prior to its acquisition by Sanofi. She began her career at Skadden, Arps, Slate, Meagher & Flom LLP, where she specialized in mergers and acquisitions and securities law. Ms. Gilbert currently serves on the board of directors, including as chair of the nominating and corporate governance committee and a member of the audit committee, of each of Vaxcyte, Inc., a biopharmaceutical company developing vaccines for infectious disease targets, and CytomX Therapeutics, Inc., a biopharmaceutical company focused on the development of therapeutic cancer treatments. Ms. Gilbert previously served on the board of directors forof Dermira, Inc. (acquired by Eli Lilly and Company), a commercial-stage company focused on medical dermatology, drugs, and Achaogen, Inc. (acquired by Cipla), a commercial-stage biopharmaceutical company that developed novel antibacterial therapies. Prior to joining us, Ms. Gilbert held various roles at Ironwood Pharmaceuticals, where she served as Senior Vice President for Corporate Development and Chief Administrative Officer from March 2019 to February 2020 and oversaw corporate and business development, legal, compliance and government affairs. From February 2014 to April 2019, she served as Ironwood Pharmaceutical’s Senior Vice President and Chief Legal Officer, prior to which she served as Vice President and General Counsel. Prior to joining Ironwood Pharmaceuticals, Ms. Gilbert was Vice President, Deputy General Counsel at Cubist Pharmaceuticals, Inc. and previously served as a corporate counsel at Genzyme Corp., prior to its acquisition by Sanofi. She began her career at Skadden, Arps, Slate, Meagher & Flom LLP, where she specialized in mergers and acquisitions and securities law. Ms. Gilbert holds a B.A. in Political Science from Tufts University and J.D. from Northwestern University School of Law. We believe that Ms. Gilbert is qualified to serve on our board of directors because of her significant experience inlaunching new medicines and her in-depth knowledge of legal matters, corporate and business development, compliance and government affairs andfrom her extensive biopharmaceutical industry experience.
Ricky Sun, Ph.D.Keith R. Leonard, Jr., has served as a member of our board of directors since August 2018. Dr. Sun joined Bain Capital Life Sciences in 2016,September 2021. Mr. Leonard is chairman of the board of Unity Biotechnology where he is a Managing Director. From August 2013 to July 2016, he held various positions at Biogen Inc., including Director of Corporate Development and Strategy from January 2015 to July 2016. Prior to Biogen, Dr. Sun served as a Vice Presidentchief executive officer from 2016 to 2020, and currently serves on the board of robotic surgery pioneer Intuitive Surgical. Previously, Mr. Leonard served as the chief executive officer of Kythera Biopharmaceuticals from its founding in 2005 to its acquisition by Allergan plc in 2015. Before Kythera, Mr. Leonard spent 13 years at BlackRock, Inc.,Amgen, ultimately as a membersenior vice president and general manager of the Fundamental Equity division of BlackRock’s Alpha Strategies Group and senior analyst for BlackRock’s Fundamental Large Cap Growth equity team, covering the health care sector.Amgen Europe, where he ran all commercial operations in 28 countries. Prior to that heposition, Mr. Leonard ran Amgen’s manufacturing operations in Europe, established Amgen’s presence in inflammation, served as head of information management, and had leadership roles in sales and marketing, engineering, operations, and finance. Mr. Leonard previously served as an independent director of Sanifit Laboratories SA (a privately held clinical-stage biopharmaceutical company), Sienna Biopharmaceuticals, Inc., Anacor Pharmaceuticals, Inc., Affymax, Inc., and ARYx Therapeutics, and was a senior healthcare analyst at Citadel LLC and Alyeska Investment Group, L.P., in Chicago and worked asventure partner with ARCH Venture Partners. He holds a pharmaceuticals equity research analyst on Wall Street, spending time at Lehman Brothers and Morgan Stanley. Dr. Sun received a Ph.D. degree in Chemistry and Chemical BiologyMaster of Business Administration from Harvard University, an MBA from New York University Sternthe Anderson School of BusinessManagement, University of California, Los Angeles, a Master of Science in mechanical engineering from University of California, Berkeley, a Bachelor of Arts in history from University of Maryland, College Park, and a B.A.Bachelor of Science in Chemistryengineering from Berea College.University of California, Los Angeles. We believe that Dr. Sun’s life sciences investment experience qualifies himMr. Leonard is qualified to serve on ourthe board of directors.directors because of his years of experience as an executive in the pharmaceutical industry, his expertise in dermatology and deep commercial expertise.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE
FOR THE ELECTION OF EACH OF THE ABOVE NAMED NOMINEES
Directors Continuing in Office Until the 20222025 Annual Meeting of Stockholders
Bhaskar Chaudhuri, Ph.D. has served as a member of our board of directors since April 2016, has served as the Chair of the Compensation Committee since 2020 and is one of our co-founders. Since June 2011, he has been the Operating Partner at Frazier Healthcare Ventures. Prior to that time, Dr. Chaudhuri served as President of Valeant Pharmaceuticals International, Inc. (currently Bausch Health) from January 2009 to September 2010. Prior to joining Valeant, Dr. Chaudhuri served for seven years as President and Chief Executive Officer of Dow Pharmaceutical Sciences, Inc. and as a member of its board of directors from 2003 to 2008, at which time Dow was acquired by Valeant. Prior to that, Dr. Chaudhuri served as Executive Vice President of Scientific Affairs at Bertek Pharmaceuticals, Inc., a subsidiary of Mylan N.V., from September 2000 to March 2002. Prior to his position at Bertek, Dr. Chaudhuri served as the General Manager of the Dermatology Division of Mylan from September 1998 to August 2000. Dr. Chaudhuri joined Mylan through the acquisition of Penederm, Inc., where he worked
from 1992 to 1998 in a number of senior positions before becoming the Vice President of Research and Development. Dr. Chaudhuri serves on the boards of directors of
Teligent, Tarsus Pharmaceutical, Inc., and previously served on the board of directors of Teligent, Inc. and Corium International, Inc. He also serves on the Advisory Board of the Johns Hopkins Berman Institute of Bioethics. Dr. Chaudhuri received a B.S. in Pharmacy and a M.S. in Industrial Pharmacy from Jadavpur University and a Ph.D. in Pharmaceutics from the University of Louisiana. We believe Dr. Chaudhuri is qualified to serve on our board of directors because of expertise in dermatology and his many years of experience in the pharmaceutical industry, including his prior positions in senior executive roles at major pharmaceutical companies.
Howard G. Welgus, M.D. has served as a member of our board of directors since August 2020. Dr. Welgus served as our Chief Medical Officer from April 2017 to July 2020. From February 2016 to June 2018, Dr. Welgus served as the Chief Medical Officer at Verrica Pharmaceuticals Inc. Prior to joining Verrica, Dr. Welgus served as the Chief Medical Officer at Thesan Pharmaceuticals Inc. from September 2012 to November 2016 and served as the Chief Medical Officer at Nycomed US Inc. from May 2009 to November 2010. From 1999 to 2009, he served as the Vice President and head of the Dermatology and Inflammation therapeutic areas in Discovery at Pfizer Inc. in Ann Arbor, MI. Prior to joining the private sector, Dr. Welgus was a faculty member at Washington University for 17 years. Dr. Welgus is a board-certified dermatologist and received a M.D. from Washington University School of Medicine in St. Louis and a B.A. in Biology from Rice University. We believe that Dr. Welgus is qualified to serve on our board of directors because of his extensive knowledge of our business, expertise in dermatology and his experience in the biopharmaceutical industry.
Jonathan Silverstein, J.D.Sue-Jean Lin has served as a member of our board of directors since August 2018. Mr. Silverstein is currentlyJune 2021 and has served as the Chair of the Audit Committee since September 2022. Since 2018, Ms. Lin has served as a Managing PartnerSenior Vice President and Chief Information Officer, and in 2021 expanded her role to Chief Information and Transformation Officer, at Alcon, a global leader in eye care where she played a key role during its journey to becoming an independent, publicly traded company. From 2016 to 2018, she served as a member of the Hill-Rom executive leadership team, serving in the capacity of senior vice president and chief information officer. Here she was instrumental in developing a new business model that enabled patient engagement and improved the effectiveness of biomedical professionals. From 1989 to 2015, she also served multiple roles, including as the senior vice president and chief information officer, and as the regional chief financial officer for Europe, Middle East, Africa, and Asia Pacific commercial operations at Allergan, plc, a public pharmaceutical company that was acquired by AbbVie Inc. in 2020. Ms. Lin holds a bachelor’s degree in accounting and a Co-Head of Global Private Equity at OrbiMed Advisors LLC, an investment firm. Mr. Silverstein currently serves on the boards of directors of several private companies. Mr. Silverstein previously served on the boards of directors of several public companies, including Adicet Bio, Inc., Ascendis Pharma A/S, Audentes Therapeutics, Inc., Avedro, Inc., Glaukos Corporation, Intercept Pharmaceuticals Inc., resTORbio Inc., Rhythm Pharmaceuticals, Inc., scPharmaceuticals Inc., and Sorrento Tech, Inc. (formerly known as Roka BioScience, Inc.). Previously, Mr. Silverstein was a director of life sciencesmaster’s degree in the investment banking department at Sumitomo Bank. He also received a B.A. from Denison University and a J.D. and M.B.A.business administration from the University of San Diego.Nevada, Reno. We believe that Mr. Silverstein’s strategic development and capital markets experience qualifies him to serve on our board of directors.
Directors Continuing in Office Until the 2023 Annual Meeting of Stockholders
Patrick Heron has served as the Chairman of our board of directors since December 2019, and has been a member of our board of directors since April 2016. Since September 1999, Mr. Heron has been a managing general partner with Frazier Healthcare Partners, where he has been active in company formations and initial investments in various biotechnology companies, including Marcadia Biotech Inc., Calixa Therapeutics, Inc. and VentiRx Pharmaceuticals, Inc. He also led Frazier’s involvement in MedPointe Inc. Prior to joining Frazier, Mr. Heron helped develop McKinsey & Company’s west coast biotechnology consulting practice. Mr. Heron currently serves on the board of directors of Mirum Pharmaceuticals, Inc. and Iterum Therapeutics plc. He previously served on the boards of directors of the Tobira Therapeutics, Inc. and Collegium Pharmaceuticals, Inc. Mr. Heron received a B.A. in Political Science from the University of North Carolina at Chapel Hill and an M.B.A. from Harvard Business School. We believe that Mr. HeronMs. Lin is qualified to serve on our board of directors because of his investing and operations experiences in the life sciences industry.
Joseph L. Turner was elected to become a member of our Board of Directors and Chairman of the Audit Committee in January 2020 upon the effectiveness of our initial public offering. Mr. Turner currently serves on the board of directors and is the chair of the audit committee of Miragen Therapeutics, Inc. Previously, Mr. Turner served as a director and chair of the audit committee of Sophiris Bio Inc., Corcept Therapeutics, Inc., Alexza Pharmaceuticals, Inc. and Kythera Biopharmaceuticals, Inc. Prior to retiring from active employment in 2006, Mr. Turner served as Chief Financial Officer at Myogen, Inc. from 1999 until it was acquired by Gilead Sciences, Inc. in 2006, and previously served as the Chief Financial Officer at Centaur Pharmaceuticals, Inc. and Chief Financial Officer and Vice President, Finance and
Administration at Cortech, Inc. Mr. Turner has an M.B.A. from the University of North Carolina at Chapel Hill, an M.A. in molecular biology from the University of Colorado, and a B.A. in chemistry from Swarthmore College. We believe that Mr. Turner possesses specific attributes that qualify him to serve as a member of our board of directors, including his years ofher experience in the biotech and pharmaceutical industries and his financial sophistication and expertise.
Todd Franklin Watanabe has served as our President and Chief Executive Officer since April 2017. Prior to joining Arcutis Biotherapeutics, he served as co-founder and Chief Operating Officer of Kanan Therapeutics, Inc., a cardiovascular drug development company from December 2015 to February 2018, and before that, he served as Vice President of Strategy and Corporate Development at Kythera Biopharmaceuticals Inc. from October 2013 to November 2015. Mr. Watanabe was an executive at Amgen, Inc. from 2005 to 2013, where he was involved in the development of Repatha for hyperlipidemia and Aimovig for migraine, and worked on the U.S. marketing of Enbrel in both dermatology and rheumatology. Previously, he was an executive with Eli Lilly and company, and an official in the U.S. Government. He was also a commissioned officer in the U.S. Navy Reserves for 25 years. Mr. Watanabe received his M.A. in National Security Studies, and his B.A. in International Relations, both from Georgetown University. We believe that Mr. Watanabe is qualified to serve on our board of directors because of his experience with biotechnology companies, including working with and serving in varioussenior executive positions in life sciences companies.a number of industries, including healthcare, expertise in dermatology, and her financial, information technology and cybersecurity expertise.
Board Recommendation
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE ELECTION OF EACH OF THE THREE NAMED CLASS III DIRECTOR NOMINEES
a
PROPOSAL NO. 2
RATIFICATION OF SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Appointment of Independent Registered Public Accounting Firm
The Audit Committee of our Board has engaged Ernst & Young LLP (“EY”), as our independent registered public accounting firm for the year ending December 31, 2021,2023, and is seeking ratification of such selection by our stockholders at the Annual Meeting. EY has served as the Company’s independent registered public accounting firm since 2019. Representatives of EY are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our bylaws nor other governing documents or law require stockholder ratification of the selection of EY as our independent registered public accounting firm. However, the Audit Committee is submitting the selection of EY to our stockholders for ratification as a matter of good corporate practice. If our stockholders fail to ratify the selection, the Audit Committee will reconsider whether or not to retain EY. Even if the selection is ratified, the Audit Committee in its discretion may direct the appointment of a different independent registered public accounting firm at any time during the year if they determine that such a change would be in the best interests of the Company and our stockholders.
Principal Accountant Fees and Services
The following table provides information regarding the fees incurred for services related to the fiscal years ended December 31, 20202022 and 2019,2021, by EY, our independent registered public accounting firm.
| | | Year Ended December 31, | | Year Ended December 31, |
| | 2020 | | 2019 | | 2022 | | 2021 |
| Audit Fees (1) | Audit Fees (1) | $ | 640,000 | | | $ | 1,177,000 | | Audit Fees (1) | $ | 1,598,800 | | | $ | 1,047,750 | |
| Tax Fees | Tax Fees | 47,750 | | | 28,040 | | Tax Fees | 342,066 | | | 36,200 | |
| Audit-Related Fees | Audit-Related Fees | — | | | — | | Audit-Related Fees | — | | | — | |
| All Other Fees | All Other Fees | — | | | — | | All Other Fees | 1,425 | | | 4,200 | |
| Total Fees | Total Fees | $ | 687,750 | | | $ | 1,205,040 | | Total Fees | $ | 1,942,291 | | | $ | 1,088,150 | |
(1)Audit fees are for professional services rendered for the audits of our financial statements for the years ending December 31, 20202022 and 2019;2021; professional services rendered for the audit of our internal controls for the year ended December 31, 2021; reviews of quarterly financial statements; professional services rendered in connection with our registration statements and securities offerings; and other accounting and financial reporting consultation services billed as audit fees or necessary to comply with the standards of the Public Company Accounting Oversight Board (United States). Fees for 20202022 include services associated with our registration statements including“at-the market” offering program in March 2022 and our equity offering completed in August 2022. Fees for 2021 include services associated with our follow-on equity offering completed in October 2020. FeesFebruary 2021 and our “at-the-market” offering program, which we launched in 2019 include fees associated with our initial public offering, which was completed in January 2020.May 2021.
All of the services described above were pre-approved by our Audit Committee. The Committee concluded that the provision of these services by EY would not affect their independence.
Pre-Approval Policies and Procedures
The Audit Committee orhas adopted a delegatepolicy (the “Pre-Approval Policy”) that sets forth the procedures and conditions pursuant to which audit and non-audit services proposed to be performed by the independent registered public accounting firm may be pre-approved. The Pre-Approval Policy provides that annual audit services engagement terms and fees are subject to the approval of the Audit Committee pre-approves,and delegates certain authority to the Chair of the Audit Committee to approve audit, audit-related, tax or provides pursuant to pre-approvals policies and procedures for the pre-approval of, all audit andpermissible non-audit services, provided that the delegated services do not exceed 10% of the prior year actual costs for annual services provided by the independent registered public accounting
firm, as established by the Audit Committee. Any services above the 10% limit require the approval of the full Audit Committee. The Pre-Approval Policy also provides that each pre-approval decision shall be communicated to the Audit Committee at its next scheduled meeting.
The Audit Committee considers whether services proposed to be performed by the independent registered public accounting firm.firm are consistent with the SEC’s rules on auditor independence. The charter ofAudit Committee will also consider whether the independent auditor is best positioned to provide the most effective and efficient service.
In connection with the Pre-Approval Policy, the Audit Committee is available at https://investors.arcutis.com/corporate-governance/governance-overview.will monitor the audit services engagement as necessary, and will approve, if necessary, any changes in terms, conditions and fees resulting from changes in audit scope, Company structure or other items.
The Audit Committee approved allAll services to the Company provided by the Company’s independent registered public accounting firm after the adoption of the audit, audit-related, tax and other services provided by EY for 2020 and 2019 andPre-Approval Policy in 2022 were approved in accordance with the estimated costs of those services. Actual amounts billed, to the extent in excess of the estimated amounts, are periodically reviewed and approved by the Audit Committee.Pre-Approval Policy.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE
FOR “FOR” RATIFICATION OF OUR SELECTION OF ERNST & YOUNG LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM.FIRM FOR 2023.
a
REPORT OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORSREPORT
The material in this report is not “soliciting material,” is not deemed “filed” with the SEC, and is not to be incorporated by reference into any filing of Arcutis Biotherapeutics, Inc.under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended.
The primary purpose of the Audit Committee is to oversee our financial reporting processes on behalf of our Board. The Audit Committee’s functions are more fully described in its charter, which is available on our website at https://investors.arcutis.com/corporate-governance/governance-overview. Management has the primary responsibility for our financial statements and reporting processes, including our systems of internal controls.
In fulfilling its oversight responsibilities, the Audit Committee reviewed and discussed with management the Company’s audited financial statements as of and for the year ended December 31, 2020.2022. The Audit Committee has discussed with Ernst & Young LLP (“EY”), the Company’s independent registered public accounting firm, the matters required to be discussed by the applicable requirements of, Communications with Audit Committees, as adopted by the Public Company Accounting Oversight Board (“PCAOB”) and the SEC.
In addition, the audit committeeAudit Committee has received the written disclosures and the letter from EY required by PCAOB Ethics and Independence Rule 3526, “Communication with Audit Committees Concerning Independence”, and the Audit Committee has discussed with EY their independence from the Company and its management. Finally, the Audit Committee discussed with EY, with and without management present, the scope and results of EY’s audit of such financial statements.
Based on these reviews and discussions, the Audit Committee has recommended to our Board that such audited financial statements be included in our Annual Report on Form 10-K for the year ended December 31, 20202022 for filing with the SEC. The Audit Committee has selected EY as our independent registered public accounting firm for the fiscal year ending December 31, 2021,2023, and is seeking ratification of such selection by the stockholders.
| | |
| Audit Committee |
| Sue-Jean Lin, Chair |
Joseph L. Turner Chair |
Bhaskar Chaudhuri, Ph.D.Neha Krishnamohan |
| Halley Gilbert |
EXECUTIVE OFFICERS
The following is biographical information for our executive officers, including their ages as of April 3, 2023.
| | | | | | | | | | | | | | |
| Name | | Age | | Position(s) |
| Todd Franklin Watanabe | | 55 | | President, Chief Executive Officer and Director |
| Scott L. Burrows | | 46 | | Senior Vice President and Chief Financial Officer |
| David W. Osborne, Ph.D. | | 62 | | Senior Vice President and Chief Technical Officer |
| Patrick E. Burnett, M.D., Ph.D. | | 51 | | Senior Vice President and Chief Medical Officer |
| Ken A. Lock | | 49 | | Senior Vice President and Chief Commercial Officer |
| Patricia A. Turney | | 56 | | Senior Vice President, Operations |
| Matthew R. Moore | | 50 | | Senior Vice President and Chief Business Officer |
| Masaru Matsuda, JD | | 53 | | Senior Vice President and General Counsel |
Executive Officers
Mr. Watanabe’s biographical information is included above under “Proposal No. 1 Election of Directors.”
Scott L. Burrows has served as our Chief Financial Officer since April 1, 2021. Mr. Burrows previously served as our Vice President of Finance from May 2019 to April 2021. Prior to joining Arcutis, he was the Head of International Investor Relations for Shire Plc in Zug, Switzerland from March 2018 to May 2019. Previously, he spent 15 years at Amgen Inc. in various finance roles of increasing responsibility, including Financial Planning & Analysis, Treasury, and Investor Relations commencing in 2003. Mr. Burrows started his career as a management consultant with Arthur Andersen in Los Angeles, California. He received both his M.B.A. and B. A. in Business Economics from UCLA and is a Certified Public Accountant (inactive).
David W. Osborne, Ph.D. has served as our Chief Technical Officer since April 2017 and is one of our cofounders. From April 2008 to May 2016, Dr. Osborne held various positions at Tolmar Inc., including Chief Scientific Officer from December 2013 to May 2016. Prior to joining Tolmar, Dr. Osborne served as Vice President of Product Development at Dow Pharmaceutical Sciences, Inc. from September 2003 to March 2008 and at Atrix Laboratories, Inc. through its acquisition of ViroTex Corp. from 1999 to 2003. He started his career as a formulation group leader at The Upjohn Company and as a Group Leader, Skin Care at Calgon Vestal Laboratories, a subsidiary of Merck & Co., Inc. Dr. Osborne received a B.S. in Chemistry from Missouri State University and a Ph.D. in Physical Chemistry from Missouri University of Science and Technology.
Patrick E. Burnett, M.D., Ph.D. has served as our Chief Medical Officer since August 2020. Prior to that Dr. Burnett was the Chief Medical Officer at Verrica Pharmaceuticals since April 2018. Prior to that, Dr. Burnett was at Sun Pharmaceuticals where he was Associate Vice President of Clinical Development from September 2015 to March 2018, with oversight of the dermatology and rheumatology pipeline. Prior to Sun Pharmaceuticals, Dr. Burnett was at Novartis from 2010 to August 2015, most recently as Global Program Medical Director. He is a board certified dermatologist and was a member of the medical faculty at Vanderbilt University Medical Center as an Assistant Professor of Dermatology from 2004 to 2010. Dr. Burnett holds an M.D. and Ph.D. in neuroscience from Johns Hopkins School of Medicine and a B.S. in Biology and Biochemistry from the University of Iowa.
Ken A. Lock has served as our Chief Commercial Officer since October 2019. Prior to joining Arcutis, he served as the Executive Director of Sales and Marketing at Gilead Sciences, concurrently leading the Inflammation and Pulmonary Hypertension U.S. commercial franchises from December 2013 to August 2019. Prior to Gilead, Mr. Lock was employed at Amgen, Inc. from March 2007 to November 2013, where he was involved in the prelaunch global development of Repatha for hyperlipidemia and also held U.S. brand marketing and sales leadership roles for Enbrel for Rheumatoid Arthritis and Psoriasis. From June 2003 to February 2007 Mr. Lock was at Wyeth Pharmaceuticals where he held various positions including Strategic Planning, International Commercial Operations, and Marketing for Enbrel in both Rheumatology and Dermatology. He started his career in process development and biologics manufacturing at IDEC Pharmaceuticals in 1996. Mr. Lock received both his B.S. in Biochemistry / Cell Biology and B.A. in Psychology from University of California, San Diego and completed his M.B.A at Cornell University.
Patricia A. Turney joined Arcutis as our Senior Vice President of Operations in November 2019. Prior to joining Arcutis, she was Vice President, External Supply at Amgen Inc., where she was responsible for the manufacture of over $5B in annual product sales, approximately 300 raw material & device suppliers, and 55 contract manufacturing sites spanning 10 countries. Previously, Ms.Turney led Amgen’s Manufacturing Site Operations in The Netherlands, supplying patients in over 75 countries. Ms. Turney held a wide variety of roles with increasing responsibility during her 23 years tenure at Amgen: including R&D, Manufacturing, Facilities & Engineering, EH&S, and Quality. She received her B.S. in Mathematics and Engineering from the US Naval Academy, and her M.B.A. from UCLA’s Anderson School of Management. Prior to her career at Amgen, Ms. Turney was a U.S. Naval Aviator and served in the US Navy in various locations around the world.
Matthew R. Moore joined Arcutis as Chief Business Officer in January 2021. Mr. Moore has over 20 years of strategy, transaction and operations experience in the biopharmaceutical industry. Most recently, he served as Vice President, Corporate Business Development and Alliance Management at Allergan, where he led worldwide strategy and business development for the company’s $4B+ Medical Aesthetics business unit. During his tenure at Allergan and its predecessor companies, Actavis and Forest Labs, Mr. Moore was responsible for creating and executing business development growth strategies across multiple therapeutic areas including medical aesthetics, neuroscience, anti-infectives and hospital products. In addition, Mr. Moore served as a key deal team member in Actavis’ transformational acquisition of Allergan and Allergan’s ultimate sale to AbbVie. Prior to Allergan, Mr. Moore held executive roles at DOV Pharmaceutical and he started his career in the healthcare investment banking group at CIBC Oppenheimer. Mr. Moore earned his B.A. in Psychology from Trinity College.
Masaru Matsuda, JD joined Arcutis as General Counsel and Corporate Secretary in January 2022. Mr. Matsuda previously served as Senior Vice President, General Counsel, Chief Compliance Officer, and Corporate Secretary at Halozyme Therapeutics, Inc. Prior to Halozyme, Mr. Matsuda worked at Amgen for 18 years in positions of increasing responsibility with his last role serving as Vice President, Law, Global Commercial Operations, where he was responsible for strategic commercial legal support to the US Commercial Operations organization, as well as Medical Affairs, Compliance, Global Value, Access & Policy, Global Marketing, and Biosimilars divisions. Mr. Matsuda received a B.S. in Business Administration with a dual emphasis in Corporate Finance and International Finance from the University of Southern California, and J.D. from University of California College of the Law, San Francisco.
CORPORATE GOVERNANCE
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The codeCode of business conductBusiness Conduct and ethicsEthics is available on our website at https://investors.arcutis.com/corporate-governance/governance-overview. The Audit Committee and Nominating and Corporate Governance Committee are responsible for the development and periodic review of the Code of Business Conduct and Ethics. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website. The reference to our web address does not constitute incorporation by reference of the information contained at or available through our website.
Corporate Governance Guidelines
We believe in sound corporate governance practices and have adopted formal Corporate Governance Guidelines to enhance our effectiveness. Our Board adopted these Corporate Governance Guidelines in order to ensure that it has the necessary practices in place to review and evaluate our business operations as needed and to make decisions that are independent of our management. The Corporate Governance Guidelines are also intended to align the interests of directors and management with those of our stockholders. The Corporate Governance Guidelines set forth the practices our Board follows with respect to Board and committee composition and selection, Board meetings, Chief Executive Officer performance evaluation and succession planning. A copy of our Corporate Governance Guidelines is available on our website at https://investors.arcutis.com/corporate-governance/governance-overview.
Pledging and Hedging Policies
We maintain an Insider Trading Compliance Policy that prohibits our employees, officers and directors from engaging in hedging or similar monetization transactions, including put options, call options, short sales and exchange fund transactions. Our policy also prohibits employees, officers and directors from using or pledging securities as collateral in margin accounts or for loans unless approved by our designated compliance officer under the policy.
Independence of the Board of Directors
As required under the Nasdaq Global Select Market (“Nasdaq”) rules and regulations, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by such board. The Board consults with the Company’s counsel to ensure that the Board’s determinations are consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent Nasdaq listing standards, as in effect from time to time.
Consistent with these considerations, our Board has determined that all of our directors, other than Mr. Watanabe and Dr. Welgus, qualify as “independent” directors in accordance with the Nasdaq listing requirements. Mr. Watanabe is not considered independent because he is an employee of Arcutis Biotherapeutics, Inc.. Dr. Welgus is not considered independent because he has been an employee of Arcutis Biotherapeutics, Inc. within the last three years. The Nasdaq independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his or her family members has engaged in various types of business dealings with us. In addition, as required by Nasdaq rules, our Board has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our Board considered information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
As required under Nasdaq rules and regulations, our independent directors meet in regularly scheduled executive sessions at which only independent directors are present. Each of the Audit Committee and Compensation Committee of our Board are comprised entirely of directors determined by the Board to be independent within the meaning of Nasdaq and SEC rules and regulations applicable to the members of such committees. With the exception of Dr. Welgus, each of the members of our Nominating and Corporate Governance Committee is an independent director under the applicable rules and regulations of Nasdaq relating to Nominating and Corporate Governance Committee independence. As a result, in accordance with Nasdaq Rule 5605(e), director nominees are selected, or recommended for the Board’s selection, by the independent directors constituting a majority of the Board’s independent directors in a vote in which only independent directors participate.
Leadership Structure of the Board
Our bylawsBylaws and Corporate Governance Guidelines provide our Board with flexibility to combine or separate the positions of Chairman of the Board and Chief Executive Officer and/or to implement a lead director in accordance with its determination that utilizing one or the other structure would be in the best interests of the Company. Mr. Heron currently serves as the Chairman of our Board. In that role, Mr. Heron presides over the executive sessions of the Board and as a liaison between management and the board of directors. All of our directors are encouraged to make suggestions for agenda items and pre-meeting materials for meetings of the Board of Directors.
Our Board has concluded that our current leadership structure is appropriate at this time. However, our Board will continue to periodically review our leadership structure and may make such changes in the future as it deems appropriate.
Role
Independence of the Board of Directors
As required under the Nasdaq Global Select Market (“Nasdaq”) rules and regulations, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by such board. The Board consults with the Company’s counsel to ensure that the Board’s determinations are consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in Risk Oversight Processpertinent Nasdaq listing standards, as in effect from time to time.
Risk assessment and oversight are an integral partConsistent with these considerations, our Board has determined that eight of our governance and management processes. Our Board encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings, and conducts specific strategic planning and review sessions duringten directors qualify as “independent” directors in accordance with the year that include a focused discussion and analysisNasdaq listing requirements. Mr. Watanabe is not considered independent because he is an employee of the risks facingCompany. Dr. Welgus served as our Chief Medical Officer from April 2017 to July 2020 and, as a result, is not considered independent because he was an employee of the Company within the last three years. The Nasdaq independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his or her family members has engaged in various types of business dealings with us. ThroughoutFollowing July 2023, which is the three year senior management reviews these risksanniversary of his retirement as our Chief Medical Officer, we expect Dr. Welgus to qualify as an independent director in accordance with the Board at regular Board meetingsNasdaq listing requirements. In addition, as part of management presentations that focus on particular business functions, operations or strategies and presents the steps takenrequired by management to mitigate or eliminate such risks.
Our Board does not have a standing risk management committee, but rather administers this oversight function directly throughNasdaq rules, our Board has made a subjective determination as a whole, as well as through various standing committeesto each independent director that no relationships exist, which, in the opinion of our Board, that address risks inherentwould interfere with the exercise of independent judgment in their respective areascarrying out the responsibilities of oversight. Whilea director. In making these determinations, our Board is responsible for monitoringconsidered information provided by the directors and assessing strategic risk exposure,us with regard to each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
As required under Nasdaq rules and regulations, our independent directors meet in regularly scheduled executive sessions at which only independent directors are present. Each of the Audit Committee is responsible for overseeingand Compensation Committee of our major financial risk exposuresBoard are comprised entirely of directors determined by the Board to be independent within the meaning of Nasdaq and SEC rules and regulations applicable to the stepsmembers of such committees. With the exception of Dr. Welgus, each of the members of our management has taken to monitor and control these exposures. The Audit Committee also monitors compliance with legal and regulatory requirements and considers and approves or disapproves any related person transactions. Our Nominating and Corporate Governance Committee monitorsis an independent director under the effectivenessapplicable rules and regulations of our corporate governance guidelinesNasdaq relating to Nominating and considersCorporate Governance Committee independence. As a result, in accordance with Nasdaq Rule 5605(e), director nominees are selected, or recommended for the Board’s selection, by the independent directors constituting a majority of the Board’s independent directors in a vote in which only independent directors participate.
Board Diversity and approves or disapproves any related person transactions where necessary. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs hasSkills
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Chaudhuri | Curran | Gilbert | Heron | Leonard | Lin | Krishnamohan | Turner(1) | Watanabe | Welgus |
| Skills & Expertise |
| Executive Management | ● | ● | ● | | ● | ● | ● | ● | ● | ● |
| Served as CEO or other senior executive of an organization |
| Prior Board Experience | ● | ● | ● | ● | ● | | | ● |
| |
| Experience as a director of another public company |
| Veteran | | | | | ● | | | | ● | |
| Experience serving as a member of the U.S. military, naval or air services |
| Dermatology | ● | ● | ● | ● | ● | ● | | | ● | ● |
| Prior significant business experience in dermatology |
| Finance & Accounting Expertise | | | ● | ● | | ● | ● | ● | | |
| Experience in the preparation and review of financial statements and financial reports |
| Commercialization Expertise | ● | ● | ● | | ● | | | | ● | ● |
| Experience managing the successful commercialization of products |
| Information Technology | | | | | ● | ● | | | | |
| Oversight of or significant background working with information technology systems, data management, and/or cybersecurity risks |
| Legal / Compliance Expertise | | | ● | | | | ● | ● | | |
| Experience in dealing with complex legal and public company governance issues |
| Gender |
| Gender | M | F | F | M | M | F | F | M | M | M |
| Race / Ethnicity / Nationality |
| African American / Black | | | | | | | | | | |
| Alaskan Native or Native American | | | | | | | | | | |
| Asian | ● | | | | | ● | ● | | ● | |
| Hispanic / Latinx | | | | | | | | | | |
| Native Hawaiian or Pacific Islander | | | | | | | | | | |
| White (not Hispanic or Latinx origins) | | ● | ● | ● | ● | | | ● | ● | ● |
| Two or more Races or Ethnicities | | | | | | | | | ● | |
| LGBTQ+ | | | | | | | | | | |
(1) Mr. Turner will not stand for reelection at the potential to encourage excessive risk-taking.Annual Meeting.
Board Committees
Our Board has the following standing committees: an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. Our Board may establish other committees to facilitate the management of our business. The composition and functions of each committee are described below.
Audit Committee
Our Audit Committee oversees our corporate accounting and financial reporting process. Among other matters, the Audit Committee is responsible for:
•selecting an our independent registered public accounting firm;
•the qualifications, independence and performance of our registered public accounting firm;
•the preparation of the audit committee report to be included in our annual proxy statement;
•our compliance with legal and regulatory requirements;
•our accounting and financial reporting processes, including our financial statement audits and the integrity of our financial statements; and
•reviewing and approving related-person transactions.transactions; and
•our information technology and information security programs.
The current members of our Audit Committee are Sue-Jean Lin, Neha Krishnamohan, Joseph L. Turner Bhaskar Chaudhuri, Ph.D. and Halley Gilbert. Mr. TurnerMs. Lin serves as the Chair of the committee. All members of our Audit Committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq. Our Board has determined that each of Bhaskar Chaudhuri, Halley Gilbert andSue-Jean Lin, Neha Krishnamohan, Joseph L. Turner and Halley Gilbert are an “audit committee financial expert” as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of Nasdaq. Under the rules of the SEC, members of the audit committee must also meet heightened independence standards. Our Board has determined that each of Ms. Lin, Ms. Krishnamohan, Mr. Turner Dr. Chaudhuri and Ms. Gilbert are independent under the applicable rules of the SEC and Nasdaq.
The Audit Committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq. A copy of the Audit Committee charter is available to security holders on the Company’s website at https://investors.arcutis.com/corporate-governance/governance-overview.
Compensation Committee
Our Compensation Committee oversees policies relating to compensation of and benefits for our officers and employees. Among other things, our Compensation Committee is responsible for:
•evaluating, recommending, approving and reviewing executive officer compensation arrangements, plans, policies and programs;
•evaluating and recommending non-employee director compensation arrangements for determination by our board of directors;
•administering our cash-based and equity-based compensation plans; and
•overseeing our compliance with regulatory requirements associated with the compensation of directors, officers and employees.
The current members of our Compensation Committee are Bhaskar Chaudhuri, Ph.D., Patrick Heron and Jonathan Silverstein.Keith Leonard. Dr. Chaudhuri serves as the Chair of the committee. Each of the members of our Compensation Committee is independent under the applicable rules and regulations of Nasdaq and is a “non-employee director” as defined in Rule 16b-3 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).Act.
Our Compensation Committee has retained Pay Governance LLC (“Pay Governance”), a compensation consulting firm, to serve as its independent compensation consultant and to conduct market research and analysis on our various executive positions, to assist the committee in developing appropriate incentive plans for our executives on an annual basis, to provide the committee with advice and ongoing recommendations regarding material executive compensation decisions, and to review compensation proposals of management. Pay Governance reports directly to the Compensation Committee and does not provide any non-compensation related services to the Company. The Compensation Committee reviewed the independence of Pay Governance, employing the independence factors specified in the listing requirements of Nasdaq. Based on this assessment, the Compensation Committee determined that the engagement of Pay Governance does not raise any conflicts of interest or similar concerns. In addition, the Compensation Committee evaluated the independence of its other outside advisors to the Compensation Committee, including outside legal counsel, considering the same independence factors and concluded their work for the Compensation Committee does not raise any conflicts of interest.
The Compensation Committee operates under a written charter that satisfies the applicable standards of the SEC and The Nasdaq rules. A copy of the Compensation Committee charter is available to security holders on the Company’s website at https://investors.arcutis.com/corporate-governance/governance-overview.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee is responsible for identifying and considering candidates for directorships and the size and composition of our Board, overseeing the process of evaluating the performance of our Board, evaluating and overseeing our environmental, social and governance (“ESG”) principles, initiative and risks and reporting and advising our Board on other corporate governance matters. For example, the Nominating and Corporate Governance Committee conducts a periodic review and oversees the Company’s strategy and initiatives regarding the Company’s ESG responsibility, and reports its findings to the Board on an annual basis.
The current members of our Nominating and Corporate Governance Committee are Ricky Sun, Ph.D.,Sue-Jean Lin, Terrie Curran and Howard Welgus, M.D. Dr. SunMs. Curran serves as the Chair of the committee. With the exception of Dr. Welgus, each of the members of our Nominating and Corporate Governance Committee is an independent director under the applicable rules and regulations of Nasdaq relating to Nominating and Corporate Governance Committee independence. As a result, in accordance with Nasdaq Rule 5605(e), director nominees are selected, or recommended for the Board’s selection, by the independent directors constituting a majority of the Board’s independent directors in a vote in which only independent directors participate. In carrying out its responsibilities, the Nominating and Corporate Governance Committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq rules. A copy of the Nominating and Corporate Governance Committee charter is available to security holders on the Company’s website at https://investors.arcutis.com/corporate-governance/governance-overview.
Our Nominating and Corporate Governance Committee is responsible for reviewing with the Board, on an annual basis, the appropriate characteristics, skills and experience required for the Board as a whole and its individual members. The Nominating and Corporate Governance Committee, in evaluating the suitability of individual candidates (both new candidates and current members), the independent directors of our Board, in recommending candidates for election, and the Board, in approving (and, in the case of vacancies, appointing) such candidates, may take into account many factors, including but not limited to the following:
•independence;
•personal and professional integrity;
•experience in corporate management, such as serving as an officer or former officer of a publicly held company;
•experience in the life sciences and biotechnology fields;
•experience as a board member or executive officer of another publicly held company;
•diversity, including diversity of expertise and experience in substantive matters pertaining to our business relative to other board members;
•conflicts of interest; and
•practical and mature business judgment.
Currently, our Board evaluates each individual in the context of the Board as a whole, with the objective of assembling a group that can best maximize the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas. Our Board will consider director candidates recommended by stockholders. For a stockholder to make any nomination for election to the Board at an annual meeting, the stockholder must provide notice to the Company, which notice must be delivered to, or mailed and received at, the Company’s principal executive offices not less than 90 days and not more than 120 days prior to the one-year anniversary of the preceding year’s annual meeting; provided, that if the date of the annual meeting is more than 30 days before or more than 30 days after such anniversary date, the stockholder’s notice must be delivered, or mailed and received, not earlier than 120 days prior to the date of the annual meeting and not later than 90 days prior to the date of the annual meeting or, if later, the 10th day following the date on which public disclosure of the date of such annual meeting is made. Further updates and supplements to such notice may be required at the times, and in the forms, required under our bylaws. As set forth in our bylaws, submissions must include information regarding the proposed nominee that is required to be disclosed in a proxy statement or other filings in a contested election pursuant to Section 14(a) under the Exchange Act, (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected). Our bylaws also specify further requirements as to the form and content of a stockholder’s notice. We recommend that any stockholder wishing to make a nomination for director review a copy of our bylaws, as amended and restated to date, which is available, without charge, from our Corporate Secretary, at 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361.
Meetings of the Board of Directors, Board and Committee Member Attendance and Annual Meeting Attendance
Our Board met thirteensix times acted by unanimous written consent eight times, and acted by email consent twice during 2020.2022. The Audit Committee met fivesix times. The Compensation Committee met three times and acted by email consent twice.four times. The Nominating and Corporate Governance Committee met three times and did not act by unanimous written consent.four times. During 2020,2022, each Board member attended at least 75% of the meetings of the Board and of the committees of the Board on which he or she served, in each case, to the extent appointed as a Board member at the relevant time of each meeting. We encourage all of our directors and nominees for director to attend our annual meeting of stockholders; however, attendance is not mandatory.
Role of Board in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our Board encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings, and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the Board at regular Board meetings as part of management presentations that focus on particular business functions, operations or strategies and presents the steps taken by management to mitigate or eliminate such risks.
Our Board does not have a standing risk management committee, but rather administers this oversight function directly through our Board as a whole, as well as through various standing committees of our Board that address risks inherent in their respective areas of oversight. In late 2022, we completed an Enterprise-wide risk assessment to identify areas that identified the primary risks that could impede us from achieving our strategic objectives. The risk assessment followed a disciplined approach and included interviews with senior leadership to identify and assess the significance of enterprise-wide risks at the Company. Such identification and assessment facilitates planning and accountability on how to manage and ameliorate such risks. The assessment was reviewed with the Audit Committee in early 2023 and will be updated at least annually. While our Board is responsible for monitoring and assessing strategic risk exposure, our Audit Committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures. The Audit Committee also monitors compliance with legal and regulatory requirements and considers and approves or disapproves any related person transactions. Our Nominating and Corporate Governance Committee monitors the effectiveness of our corporate governance guidelines and considers and approves or disapproves any related person transactions where necessary. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Our Environmental, Social and Governance Principles
We believe in operating in an ethical, socially conscious, and environmentally sustainable manner and follow these principles to better server our patients, employees, and all our stakeholders. From the initial founding of Arcutis, our business and people have strived to create an environment where diversity, inclusion, innovation and sustainability are integrated into all aspects of our operations. This is evidenced by the treatments we have developed to meet unmet needs of dermatology clinicians and individuals impacted by immune-mediated skin disease, the diverse talent of our employees, our strong commitment to our community and the environment, and our diverse and experienced Board.
Governance
Board oversight of our ESG program is provided by the Nominating and Corporate Governance Committee. Their charter includes evaluating and overseeing our ESG principles, initiatives, and risks. Where appropriate, ESG matters are also discussed within the Audit and Compensation committees and annually an ESG report is provided to the Board. Internal oversight and execution is managed with a cross-functional team led by our Head of ESG, and includes our General Counsel, Vice President of Human Resources, Head of Corporate Communications, and Head of Investor Relations.
In 2022, we published our first ESG report, highlighting the many initiatives and successes of Arcutis within the ESG space.
Our Commitment to Diversity
We believe that the diversity of our employees supports our ability to develop innovative medicines while also making our company stronger. We value our employees and the diversity each employee brings, including, but not limited to, diversity of race, gender, age, sexual orientation, backgrounds, experiences, skills, opinions and personalities.
Gender Diversity
The charts below set forth the gender diversity of our overall workforce, leadership positions and Board, in each case as of December 31, 2022, using the gender binary.
(1) Includes employees in positions of Vice President and above.
Racial and Ethnic Diversity of Workforce in United States
In the United States, where 96% of our employees reside, 31% of our workforce self-report as non-white. For employees located outside of the United States, we do not request racial diversity data, as tracking these metrics is largely prohibited by law. The chart below sets forth the racial and ethnic diversity of our workforce in the United States as of December 31, 2022.
(1) Includes employees in positions of Vice President and above.
The categories of the racial and ethnic diversity of our workforce in the United States can be further broken down as shown in the graph below.
Pay Equity Assessment
As part of our commitment to pay equity and equality across all employees, we have committed to conducting an annual pay equity assessment to identify and address potential pay gaps. Our first assessment was conducted in 2022 for United States employees below the Senior Vice President level, and no evidence of a systemic pay equity issue was found. More information on this assessment can be found in the Compensation Discussion and Analysis section of this proxy.
Professional Development
As Arcutis continues to expand our workforce, we recognize the need to provide opportunities for our people to grow and develop professionally. We have a professional development program with a mission to support a continuous learning culture to enhance awareness, competence, performance and innovation. Our professional development program focuses on three key objectives:
| | | | | | | | |
Grow (Career Development) | Connect (Organization Effectiveness) | Lead (Leadership Training) |
•Identify personal career goals and aspirations •Promote discussion opportunities between managers and employees | •Enhance connection across a common platform for improving communication, collaboration and positive working relationships | •Support leadership development and effectiveness for leaders at all levels |
Tools used: •Personal inventory of skills and interests supported by conversation guides for managers and employees | Tools used: •Insights® Discovery individual profiles and team workshops | Tools used: •Intent Based Leadership® workshops and resources |
Our professional development program was started in 2021 and continues to evolve with the company. All Arcutis employees continue to participate in the Insights® Discovery program, as well as participate in mid-year and end-of-year performance reviews to review performance and development opportunities. In 2023, manager development courses are being implemented for all front-line managers.
In addition to these company-wide professional development activities, Arcutis is also a corporate sponsor of the Healthcare Businesswomen’s Association (“HBA”) and Women in Bio. We encourage and sponsor participation in the HBA, #IamRemarkable, and the HCL Technologies’ Women Lead mentoring program.
Arcutis Culture Team and Community Engagement
Since 2020, our Arcutis Culture Team (ACT) has been engaged to plan, coordinate and communicate ESG and employee engagement opportunities. ACT represents a cross-section of levels and functions to integrate and facilitate the education of our employees and provide opportunities to strengthen our employee community. In 2022, ACT reorganized into three sub-teams focused on Diversity, Equity, and Inclusion; Employee Engagement; and Charity and Community Engagement. 2022 also saw the founding of our company’s first employee-coordinated Connection Group, or affinity/interest group, and ended the year with three groups focused on varying interests of our employees. Throughout the year, the Company celebrated many diverse cultures and activities such as Black History Month, PRIDE month, International Women’s Day, Asian American and Pacific Islander Month, and Hispanic Heritage Month, to name a few.
We believe in supporting the communities where we work and live. In 2022, our people volunteered for over 1,200 hours to numerous organizations throughout the United States and gave monetary donations to nearly 100 separate organizations covering a wide range of philanthropic areas. In addition, we supported both national and local organizations ranging from the National Psoriasis Foundation to our local Manna food drive.
Health and Safety
We are also focused on the health, well-being and safety of our employees. Our policies seek to prevent and reduce workplace risks and injuries through various programs, projects, services, and assistance, such as ergonomic evaluation, hazard reporting, risk assessment, and first aid training. Employee safety is also supported by an access control system at all facilities and a dedicated 24/7 security team at our offices in Westlake Village. We require all work-related injuries or illnesses to be reported. This information is reviewed by management for analysis.
Access to and Affordability of Our Product
Providing access to our product is paramount to our business. Two initiatives were founded in 2022 with the commercialization of our first product. Our patient assistance program, Arcutis Cares™, provides Zoryve® (roflumilast) cream 0.3% at no cost to eligible uninsured or underinsured patients with plaque psoriasis with demonstrated financial need. ZORYVE Direct™, a patient access support program, helps commercially insured individuals with plaque psoriasis get access to start ZORYVE treatment as prescribed by their healthcare provider quickly and easily by helping them navigate the payer process, lowering the out-of-pocket cost for eligible patients, and offering programs that support staying on therapy.
Environmental
We have initiated an assessment of our greenhouse gas emissions footprint, in order to develop a methodology for collection and reporting. Through internal resources and third-party relationships, we are confident in our ability to quantify our scope 1 and 2 emissions while continuing to evaluate and understand our supply chain. We have also worked with our third-party suppliers to understand their sustainability practices. In addition, we have limited our environmental footprint by operating in a hybrid and virtual work environment. Our policies and work habits encourage environmentally conscious activities, such as reduced printing, automatic lights and automatic water taps, recycling, and the elimination of single-use items in our break room. We believe these policies have helped to reduce waste generated through our operations.
Compensation Committee Interlocks and Insider Participation
During 2022, our Compensation Committee consisted of Dr. Chaudhuri, Mr. Heron and Mr. Leonard. None of the members of our Compensation Committee has been one of our officers or employees during the past three years. With the exception of Mr. Watanabe who serves as our President, Chief Executive Officer and a director, none of our executive officers currently serve, or in the past fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our Board or Compensation Committee.
Pledging and Hedging Policies
We maintain an Insider Trading Compliance Policy that prohibits our employees, officers and directors from engaging in hedging or similar monetization transactions, including put options, call options, short sales and exchange fund transactions. Our policy also prohibits employees, officers and directors from using or pledging securities as collateral in margin accounts or for loans unless approved by our designated compliance officer under the policy.
Stockholder Communications with the Board of Directors
Should stockholders wish to communicate with the Board or any specified individual directors, such correspondence should be sent to the attention of the Corporate Secretary, at 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361. The Corporate Secretary will forward the communication to the Board members.
Compensation Committee Interlocks and Insider Participation
During 2020, our Compensation Committee consisted of Messrs. Heron and Silverstein and Dr. Chaudhuri. None of the members of our Compensation Committee has been one of our officers or employees during the past three years. With the exception of Mr. Watanabe who serves as our President, Chief Executive Officer and a director, none of our executive officers currently serve, or in the past fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our Board or Compensation Committee.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
We describe below transactions and series of similar transactions, since January 1, 2020,2022, to which we were a party or will be a party, in which:
•the amounts involved exceeded or will exceed $120,000; and
•any of our directors, executive officers or holders of more than 5% of our common stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest.
Director and Executive Officer Compensation
See “Executive Compensation” and “Director Compensation” for information regarding compensation of directors and executive officers.
Employment Agreements
We have entered into employment agreements with our executive officers. We most recently entered into a new employment agreement with Mr. Matsuda in connection with him joining us in January 2022. For more information regarding these agreements, see “Executive Compensation−Narrative to 2020 Summary Compensation TableDiscussion and Outstanding Equity Awards at 2020 Fiscal Year End.Analysis-Employment and Severance Agreements.”
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, penalties, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer. We have obtained an insurance policy that insures our directors and officers against certain liabilities, including liabilities arising under applicable securities laws. Since January 1, 2022, we have entered into new indemnification agreements in connection with the appointment of Ms. Krishnamohan to our Board and the hiring of Mr. Matsuda as our General Counsel and Corporate Secretary.
Investor Rights Agreement
We entered into an amended and restated investors’ rights agreement with the purchasers of shares of our convertible preferred stock, including entities with which certain of our directors are affiliated, which were outstanding prior to our initial public offering in January 2020 and which converted into shares of common stock in connection therewith. As of December 31, 2020,2022, the holders of approximately 25.810.6 million shares of our common stock (including 1.4 million shares issued and sold pursuant to the private placement of shares in connection with our follow-on financing) are entitled to rights with respect to the registration of their shares under the Securities Act.
Hawkeye Collaboration AgreementOther Transactions
In June 2019, we entered into a collaboration agreement, or Hawkeye Agreement, with Hawkeye Therapeutics, Inc.Participation in Follow-on Offerings
Frazier Life Sciences VIII, L.P., or Hawkeye, a related party with common ownership, for the development of onewhich holds 5% or more new applications of roflumilast. The Hawkeye Agreement grants Hawkeye an exclusive license to certain intellectual property developed under the agreement as it relates to the applications.
In consideration for their services to be performed under the Hawkeye Agreement, each of Arcutis Biotherapeutics, Inc., David W. Osborne, our Chief Technical Officer,capital stock and Bhaskar Chaudhuri,is affiliated with Patrick Heron, a member of our board of directors, purchased 995,000, 250,000 and 500,000 shares of common stock in Hawkeye, respectively, pursuant to a stock purchase agreement. Additionally, one of our stockholders, Frazier Life Sciences VIII, L.P., is a stockholder in Hawkeye, and Bhaskar Chaudhuri and Patrick Heron, each a member of our board of directors, are affiliated with Frazier Life Sciences VIII, L.P.
Participation in Initial Public Offering
Certain of our stockholders, including entities affiliated with holders of 5% or more of our capital stock and certain of our directors, purchased an aggregate of 4,353,005 shares of our common stock in our initial public offering at the initial public offering price and on the same terms as the other purchasers in our initial public offering and not pursuant to any pre-existing contractual rights or obligations.
In addition, certain friends and family of our directors or officers, and certain of our other non-executive officer employees purchased an aggregate of 111,764 shares of our common in our initial public offering at the initial public offering price in a directed share program.
Participation in Follow-on Offerings
Certain of our stockholders, including entities affiliated with holders of 5% or more of our capital stock, purchased an aggregate of 600,000250,000 shares of our common stock in our follow-on offering of common stock in October 2020August 2022 at the same price and on the same terms as the other purchasers in the follow-on offering and not pursuant to any pre-existing contractual rights or obligations.
Concurrently with the follow-on offering in October 2020, certain affiliates of OrbiMed Advisors LLC, or OrbiMed, purchased 1,400,000 shares of our common stock at the same price as the purchasers in the follow-on offering in a concurrent private placement. In connection with this concurrent private placement, we entered into a registration rights agreement, or the Registration Rights Agreement, with OrbiMed, pursuant to which we agreed to prepare and file a registration statement with the Securities and Exchange Commission within 123 calendar days after the closing of concurrent private placement for purposes of registering for resale the shares sold in the concurrent private placement and any securities directly or indirectly issued with respect to such shares by way of stock dividend, stock split or in connection with a combination of shares, recapitalization, merger, consolidation or other reorganization. We agreed to use reasonable best efforts to cause this registration statement to be declared effective as soon as practicable after the filing of such registration statement and within 175 days of the closing of the concurrent private placement. We also agreed, among other things, to indemnify the purchasers, their officers, directors, members, employees and agents, successors and assigns under the registration statement from certain liabilities and to pay all fees and expenses (excluding any legal fees of the selling holder(s), other than one legal counsel selected by OrbiMed holding a majority of the shares to be registered on their behalf, and any underwriting discounts and selling commissions) incident to our obligations under the Registration Rights Agreement. Jonathan T. Silverstein, a member of our board of directors, is affiliated with OrbiMed.
Certain of our stockholders, including entities affiliated with holders of 5% or more of our capital stock, purchased an aggregate of 1,575,000 shares of our common stock in our follow-on offering of common stock in February 2021 at the same price and on the same terms as the other purchasers in the offering and not pursuant to any pre-existing contractual rights or obligations.
Policies and Procedures for Related Party Transactions
Our Board has adopted a written related person transaction policy setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act of 1933, as amended, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our Audit Committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction with an unrelated third party and the extent of the related person’s interest in the transaction.
DIRECTOREXECUTIVE COMPENSATION
We maintain a
COMPENSATION DISCUSSION AND ANALYSIS
In this Compensation Discussion and Analysis (“CD&A”) set forth below, we provide an overview and analysis of the compensation awarded to or earned by our named executive officers (collectively, the “named executive officers” or “NEOs”) below during fiscal 2022, including the elements of our compensation program for our non-employee directors (the “Director Compensation Program”),named executive officers, material compensation decisions made under that program for fiscal 2022 and the material factors considered in making those decisions. Our named executive officers for the year ended 2022, which was initially adopted and approved by our Board in connection with our initial public offering effective January 30, 2020. We do not provide directors who are also our employees any additional compensation for their service as directors.
Pursuant to the Director Compensation Program, each non-employee director receives an annual retainer of $37,500 (the “Base Retainer”), the chairmanconsist of our Board receives an additional annual retainer of $30,000,principal executive officer, our principal financial officer, and non-employee directors who serve on one or more committees are eligible to receive the following annual committee fees (in addition to the annual retainer), based on whether they are the chair of such committee or a non-chair member:
our three other most highly compensated executive officers for fiscal year 2022 are:
| | | | | | | | |
| Name | | AmountPosition |
Base RetainerTodd Franklin Watanabe | | $37,500President and Chief Executive Officer |
Board and Committee Chair Service Premiums (in addition to Base Retainer)Masaru Matsuda, J.D. | | —Senior Vice President, General Counsel |
Board ChairPatrick Burnett, M.D., Ph.D. | | 30,000Senior Vice President, Chief Medical Officer |
Audit Committee ChairKenneth Lock | | 15,000Senior Vice President, Chief Commercial Officer |
Compensation Committee ChairScott Burrows | | 10,000 |
Nominating and Corporate Governance Committee Chair | | 8,000 |
Non-Chair Committee Service Premiums (in addition to Base Retainer) | | — |
Audit Committee | | 7,500 |
Compensation Committee | | 5,000 |
Nominating and Corporate Governance Committee | | 4,000Senior Vice President, Chief Financial Officer |
UnderMr. Matsuda commenced services as our Senior Vice President, General Counsel in January 2022.
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt following this proxy statement may differ materially from currently planned programs as summarized in this discussion.
Executive Summary
We are a commercial-stage biopharmaceutical company focused on developing and commercializing treatments for dermatological diseases with high unmet medical needs. Our current portfolio is comprised of highly differentiated topical treatments with significant potential to treat immune-mediated dermatological diseases and conditions. We believe we have built the Directorindustry's leading platform for dermatologic product development. Our strategy is to focus on validated biological targets, and to use our drug development platform and deep dermatology expertise to develop differentiated products that have the potential to address the major shortcomings of existing therapies in our targeted indications. We believe this strategy uniquely positions us to rapidly advance our goal of bridging the treatment innovation gap in dermatology, while maximizing our probability of technical success.
During 2022, we made significant progress in the development of our product candidates, the launch of our first product, and the achievement of our business goals, including the following:
•Received FDA approval of Zoryve® (roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and over;
•Commercially launched ZORYVE in the United States;
•Achieved coverage for ZORYVE under Express Scripts, Inc. national formularies;
•Launched Arcutis Cares patient assistance program for financially qualified uninsured and underinsured patients;
•Submitted supplemental new drug application (sNDA) for ZORYVE cream for the treatment of plaque psoriasis in children down to 2 years of age;
•Delivered positive topline results from atopic dermatitis pivotal phase 3 trials with roflumilast cream;
•Delivered strong seborrheic dermatitis phase 3 data with roflumilast foam;
•Delivered positive topline results from scalp and body psoriasis phase 3 trial with roflumilast foam in adults and adolescents;
•Enrolled our first patient in a Phase 1b alopecia areata study;
•Acquired Ducentis BioTherapeutics Ltd., a preclinical-stage biotechnology company focused on developing novel therapies for inflammation and autoimmune diseases;
•Following FDA approval of ZORYVE, raised over $170 million in a public stock offering;
•Received Health Canada acceptance of the New Drug Submission for roflumilast cream for the treatment of plaque psoriasis in adults and adolescents; and
•Grew our organization by 81% to over 260 employees to support our continued growth and to launch ZORYVE.
2022 Executive Compensation Program as adopted in January 2020, each non-employee director who is elected or appointedHighlights
Cash Compensation
Historically, our compensation program has consisted of relatively modest cash compensation and has emphasized equity compensation consistent with common practices for other pre-IPO companies. Subsequent to our Board will automatically receive an optioninitial public offering (IPO) in 2020, our compensation program continues to purchase 37,500 sharesemphasize equity compensation, however, in 2022 we continued our multi-step process to better align the cash compensation for our named executive officers with competitive market practices of comparable public companies. For 2022, we increased the base salaries of our common stock upon the director’s initial appointment or electioncontinuing named executive officers by approximately 3.8% to 14.5%. The largest increases were to our Board (the “Initial Director Grant”). In 2020, Ms. Curran, Ms. GilbertCEO and Mr. Turner eachto our CFO, both of whom remained below the median of our peer group.We did not increase the target bonus opportunity for any of our named executive officers for 2022.
Equity Awards
Our continuing named executive officers received an Initial Director Grant2022 annual equity awards in a mix of stock options and RSUs, at grant values below or slightly below the median of the 2022 peer group. We also awarded a new hire grant consisting of a mix of time- and performance-based long-term equity in the amountform of 37,500 shares upon their electionoptions and RSUs to our Board. In addition, each non-employee director who is serving on our Board immediately following an annual stockholder’s meeting will automatically be granted an annual option to purchase 18,750 shares of our common stock on the date of such annual stockholder’s meeting (the “Annual Director Grant”). Each option granted under the Director Compensation Program has an exercise price per share equal to the closing trading price of our common stock on the date of grant. One-third (1/3rd) of the shares subject to the Initial Director Grant will vest on each of the first three annual anniversaries of the grant date, subject to the award holder’scontinued service through each applicable vesting date. One hundred percent (100%) of the shares subject to the Annual Director Grant will vest on the earlier of the first anniversary of the grant date or the next annual stockholders’ meeting, subject to the award holder’s continued service through the applicable vesting date. All equity awards granted to our non-employee directors under the Director Compensation Program will, to the extent they are outstanding and unvested,vest in full immediately prior to the consummation of a change in control. In 2020, Dr. Chaudhuri, Dr. Estes, Mr. Heron, Mr. Silverstein and Dr. Sun each received an annual stock option grant in the amount of 24,000 shares. Also in 2020, Dr. Welgus received an annual option to purchase 12,000 shares and 9,000 RSU’sSVP, General Counsel in connection with the commencement of his service as the Company’s Chief Medical Officer, prioremployment.
Performance Results and Payouts
We had a strong year of execution across all departments and achieved our annual 2022 corporate goals at 115% of target.
Executive Compensation Philosophy and Objectives
Historically, our executive compensation program has been designed to his appointmentmotivate, reward, attract and retain high caliber management who create an inclusive and diverse environment and are deemed critical to our Board.success. The program seeks to align executive compensation with our short-and long-term
objectives, financial performance and stockholder priorities. Our compensation objectives are designed to support these goals by:
•Attracting, retaining and motivating superior executive talent;
•Providing fair and competitive compensation packages that are designed to incentivize executives to drive company performance;
•Focusing on variable compensation that rewards the achievement of short-term and long-term goals and emphasizing our commitment to pay-for-performance; and
•Aligning our executives’ interests with those of our stockholders to ensure that their compensation is meaningfully related to increasing shareholder value.
In keeping with our role as a publicly-held company, we maintain a commitment to strong corporate governance in connection with our named executive officer compensation arrangements where our Compensation Committee works with management to develop and maintain compensation frameworks that are appropriate and competitive for a public company. Our Compensation Committee works with Pay Governance, the Compensation Committee’s compensation consultant, to formalize our compensation philosophy and implement compensation arrangements that reflect that philosophy.
Our Compensation Committee reviews our executive compensation program to ensure our practices align the interests of our directors and executive officers with our stockholders. Below are features of our compensation program which the Compensation Committee believes demonstrate our commitment to link executive compensation to performance and to incentivize the creation of stockholder value.
| | | | | | | | |
| What We Do | | What We Do Not Do |
ü Emphasize performance-based, at-risk compensation. | | ý Grant uncapped cash incentives or guaranteed equity compensation. |
ü Emphasize the use of equity compensation to promote executive retention and reward long-term value creation. | | ý Guarantee executive officers annual salary increases or bonuses. |
ü Weight the overall pay mix toward long-term incentive compensation for senior executives. | | ý Provide excessive perquisites. |
ü Establish incentive compensation programs based on metrics that are aligned with our corporate strategy and designed to grow stockholder value. | | ý Provide any compensation-related tax gross-ups. |
ü Structure change in control severance payments as “double-trigger”, requiring both a change in control and an involuntary termination for payout. | |
|
ü Maintain an Insider Trading Compliance Policy that prohibits employees, officers and directors from engaging in hedging or pledging transactions. | | |
ü Maintain a Clawback Policy that allows the Company to seek to recover certain incentive compensation paid to its officers in situations where “Recoverable Events” have taken place. | | |
ü Maintain a Stock Ownership Guidelines policy to align the long-term interests of senior executives and non-employee directors with those of the Company's shareholders. | | |
ü Maintain a Compensation Committee comprised entirely of independent directors. | | |
ü Engage an independent compensation consultant to advise our Compensation Committee. | | |
ü Hold an annual say on pay stockholder vote. | | |
ü Conduct annual pay equity assessments. | | |
How We also provide reimbursementDetermine Executive Compensation
Role of the Compensation Committee, Board of Directors, and Executive Officers
The Compensation Committee oversees and recommends to our non-employee directors for their reasonable expenses incurred in attending meetings of ourthe board of directors and committeesapproval of our Board.In addition, we paid consulting fees in the amount of $3,125 to Dr. Welgus for consulting services he provided to us in addition to his service as a member of the Board in 2020.
Director Compensation Table
The following table sets forth information concerning theall compensation earned by our non-employee directors during the year ended December 31, 2020.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Fees Earned or Paid in Cash ($) | | Option Awards ($) (1) | | All Other Compensation ($) | | Total ($) |
| Bhaskar Chaudhuri, Ph.D. | | 50,417 | | 432,986 | | — | | 483,403 |
| Terrie Curran | | 6,917 | | 476,428 | | — | | 483,345 |
| Halley Gilbert | | 26,875 | | 663,566 | | — | | 690,441 |
| Patrick J. Heron | | 66,458 | (3) | 432,986 | | — | | 499,444 |
| Jonathan T. Silverstein, J.D. | | 38,958 | | 432,986 | | — | | 471,944 |
| Ricky Sun, Ph.D. | | 44,708 | | 432,986 | | — | | 477,694 |
| Joseph L. Turner | | 48,125 | | 434,752 | | — | | 482,877 |
| Howard G. Welgus, M.D. | | 17,291 | | — | | 3,125 | (5) | 20,416 |
| Daniel Estes, Ph.D. | | 25,875 | (3) | 432,986 | (2) | — | | 458,861 |
| Alexander Asam (4) | | — | | — | | — | | — |
(1)Amounts reflect the full grant date fair value of stock options granted during 2020 computed in accordance with ASC Topic 718, rather than the amounts paid to or realized by the named individual. See Note 9 of the audited financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020 for the assumptions used in calculating these amounts.
(2)Option award was forfeited upon Dr. Estes’ resignation from our Board in August 2020.
(3)Amounts paid to Frazier Healthcare Partners on behalf of Dr. Estes’ and Mr. Heron’s service as members of the Board.
(4)Board fees and option award were forgone by Mr. Asam.
(5)Amount represents consulting fees paid for services other than as a member of our Board.
As of December 31, 2020, outstanding options and restricted stock held by our current non-employee directors were as follows:
| | | | | | | | | | | | | | |
| | Shares Subject to Outstanding Options | | Shares of Restricted Stock |
| Bhaskar Chaudhuri, Ph.D. | | 123,965 | | — |
| Terrie Curran | | 37,500 | | — |
| Halley Gilbert | | 37,500 | | — |
| Patrick J. Heron | | 24,000 | | — |
| Jonathan T. Silverstein | | 24,000 | | — |
| Ricky Sun, Ph.D. | | 24,000 | | — |
| Joseph L. Turner | | 37,500 | | — |
| Howard G. Welgus, M.D. (1) | | 155,934 | | 9,000 |
(1)Dr. Welgus’ restricted stock awards were granted for his service as Chief Medical Officer, which continue to vest subject to his continued service as a member of our Board.
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
The following is biographical informationarrangements for our executive officers, including our named executive officers. The Compensation Committee meets throughout the year to discuss and review our executive compensation program, and typically makes decisions on base salary, annual bonus, and equity awards for our continuing named executive officers in the first quarter of the year. As further described below, the Compensation Committee considers market data and input from its independent compensation consultant, Pay Governance, and other advisors including in reviewing the competitiveness of individual compensation packages relative to peer company data and factors including the performance and
contributions of each named executive officer, our corporate performance and each individual’s responsibilities, experience, and criticality to our business strategy.
For our 2022 compensation program, our Chief Executive Officer provided an assessment of these factors and made recommendations to the Compensation Committee to assist in determining fiscal 2022 compensation levels for the named executive officers other than himself. While the Compensation Committee and board of directors utilized this information, the ultimate decisions regarding fiscal 2022 executive compensation were made by the Compensation Committee and board of directors in their agesown independent judgment. With respect to our Chief Executive Officer’s performance, the Compensation Committee considers input from the other independent members of the Board.
Our Compensation Committee regularly meets in executive session without management. While various members of management may attend committee meetings, none of our named executive officers was present during the Compensation Committee’s determinations regarding their own compensation.
Role of Compensation Consultant
The Compensation Committee has retained the services of Pay Governance as our independent compensation consultant. Pay Governance assists the Compensation Committee in its review of April 13, 2021.executive and director compensation practices, including the market competitiveness of compensation, executive compensation design, benchmarking with industry peers, and other technical considerations including those related to tax and accounting.
For fiscal 2022, Pay Governance advised and assisted with the following:
•Providing fair and competitive compensation packages that are designed to incentivize executives to drive company performance;
•Development of a peer group to be used in the evaluation of 2022 executive and director compensation;
•Market analysis of executive officer compensation compared to our peer group;
•Market analysis of long-term incentive compensation of our executive officers compared to our peer group; and
•Market analysis of director compensation compared to our peer group.
The Compensation Committee regularly evaluates the services Pay Governance provides and has final authority to engage and terminate their services. Our Compensation Committee has assessed Pay Governance’s independence consistent with Nasdaq listing standards and has concluded that the engagement of Pay Governance does not raise any conflict of interest.
Competitive Assessment
A key objective of our executive compensation program is to ensure that the overall compensation packages we offer our executive officers remain competitive with the packages offered by companies with which we compete for executive talent. The Compensation Committee consults with Pay Governance to develop a peer group of companies to serve as the basis for comparing our executive compensation program to the market.
2022 Peer Group
With the assistance of Pay Governance, the Compensation Committee annually reviews the composition of the peer group to account for changes in both our business and the businesses of the companies in the peer group. While referencing peer group compensation levels is helpful in determining market-competitive compensation for our named executive officers, the Compensation Committee and board of directors do not directly tie any pay elements to particular benchmarks within the peer group.
Rather, peer data is one consideration, along with employee knowledge, skills, experience, individual performance, and scope of responsibilities, among other factors.
In developing the 2022 peer group, the Compensation Committee considered several qualitative and quantitative elements. The targeted criteria set out below were guidelines, and not all companies selected met each of the selection criteria.
•Industry – biotechnology/biopharmaceutical companies, with preference for companies with dermatology-related products under development;
•Market Capitalization – companies within a market cap range of $380 million to $3.4 billion (~1/3 to 3x our valuation at the time the peer group was developed);
•Stage of Development – later stage, pre-commercial or limited revenue companies with a significant ongoing research and development spend;
•Maturity – companies completing IPOs within the five years of Arcutis’ 2020 IPO;
•Location – strike a balance between companies with Southern and Northern California locations (with other U.S. geographies included as needed);
•Headcount – between 50 and 500 employees.
Following this analysis, the Compensation Committee identified the following 17 publicly-traded, U.S.-based biotechnology/biopharmaceutical companies as our peer group to be used in reviewing executive compensation for 2022:
| | | | | | | | | | | | | | |
Name | | Age | | Position(s) |
Executive Officers2022 Peer Group | | | | |
Todd Franklin WatanabeAclaris Therapeutics | | 53Alector | | President, Chief Executive Officer and DirectorAnaptysBio |
Scott L. BurrowsArena Pharmaceuticals | | 44Atara Biotherapeutics | | Chief Financial OfficerBridgeBio Pharma |
David W. Osborne, Ph.D.Cara Therapeutics | | 60Kadmon Holdings | | Chief Technical OfficerKaruna Therapeutics |
Patrick E. Burnett, M.D., Ph.D.Kodiak Sciences | | 49Krystal Biotech | | Chief Medical OfficerKura Oncology |
Kenneth A. LockMannKind Corporation | | 47Revance Therapeutics | | Chief Commercial OfficerRocket Pharmaceuticals |
Patricia A. TurneyVanda Pharmaceuticals | | 54Y-maBs Therapeutics | | Senior Vice President, Operations |
Matthew R. Moore | | 48 | | Senior Vice President and Chief Business Officer |
AVROBIO, Corbus Pharmaceuticals and Puma Technology were removed from our peer group for 2022 as a result of sharp decreases in their market capitalizations where they were no longer in our targeted range. Viela Bio was removed from our peer group for 2022 as a result of their acquisition by Verizon in 2021. In lieu of these four companies, we have added in Aclaris Therapeutics, Alector, Arena Pharmaceuticals (acquired by Pfizer in May 2022; will be removed from future peer group) and Vanda Pharmacueticals to our 2022 peer group.
Our Compensation Committee expects to periodically review and update this peer group and to utilize Pay Governance for benchmarking and peer group analysis in determining and developing compensation packages for our named executive officers.
Elements of Executive Compensation
Historically, and for fiscal 2022, our executive compensation program consisted of the following elements, each established as part of our program in order to achieve the compensation objective specified below:
| | | | | | | | |
| Compensation Element | | Compensation Objectives Designed to be Achieved
and Key Features |
| Base Salary | | Attract and retain key talent by providing base cash compensation at competitive levels. |
| Cash-Based Annual Incentive Compensation | | Motivate and reward attainment of rigorous annual corporate performance goals identified as strategic drivers of long-term value creation. |
| Equity-Based Incentive Compensation | | Provide long-term incentives in the form of equity-based compensation to drive an ownership mentality, encourage retention, provide strong alignment with shareholders and reinforce the importance long-term shareholder value creation. |
| Severance and Other Benefits Potentially Payable upon Termination of Employment or Change in Control | | Create clarity around termination or change of control events and provide for retention of executives. |
| Health and Welfare Benefits | | Attract and retain key talent by providing a competitive benefits package. |
Our Compensation Committee has not established formal policies or guidelines for allocating compensation between annual and long-term incentive compensation, or between cash and non-cash compensation. Instead, through our compensation program, the Compensation Committee seeks to align pay and performance.
As can be seen in the graphs below, a large percentage of executive pay in 2022 was variable and “at-risk” (87% for the Chief Executive OfficersOfficer, and 74% on average for our other named executive officers), meaning that value to the executive is tied directly to corporate goals and stock price performance. In this sense, we believe we have established a pay-for-performance culture and pay program. The balance between these components may change from year to year based on corporate strategy and objectives, among other considerations.
Mr. Watanabe’s biographical information is included above under “Proposal No. 1 Election of Directors.”
Scott L. Burrows has served as our Chief Financial Officer since April 1, 2021. Mr. Burrows previously served as the our Vice President of Finance from May 2019 to April 2021. Prior to joining Arcutis, he was the Head of International Investor Relations for Shire Plc in Zug, Switzerland from March 2018 to May 2019. Previously, he spent 15 years at Amgen Inc. in various finance roles of increasing responsibility, including Financial Planning & Analysis, Treasury, and Investor Relations commencing in 2003. Mr. Burrows started his career as a management consultant with Arthur Andersen in Los Angeles, California. He received both his M.B.A. and B. A. in Business Economics from UCLA and is a Certified Public Accountant (inactive).
David W. Osborne, Ph.D. has served as our Chief Technical Officer since April 2017 and is one of our cofounders. From April 2008 to May 2016, Dr. Osborne held various positions at Tolmar Inc., including Chief Scientific Officer from December 2013 to May 2016. Prior to joining Tolmar, Dr. Osborne served as Vice President of Product Development at Dow Pharmaceutical Sciences, Inc. from September 2003 to March 2008 and at Atrix Laboratories, Inc. through its acquisition of ViroTex Corp. from 1999 to 2003. He started his career as a formulation group leader at The Upjohn Company and as a Group Leader, Skin Care at Calgon Vestal Laboratories, a subsidiary of Merck & Co., Inc. Dr. Osborne received a B.S. in Chemistry from Missouri State University and a Ph.D. in Physical Chemistry from Missouri University of Science and Technology.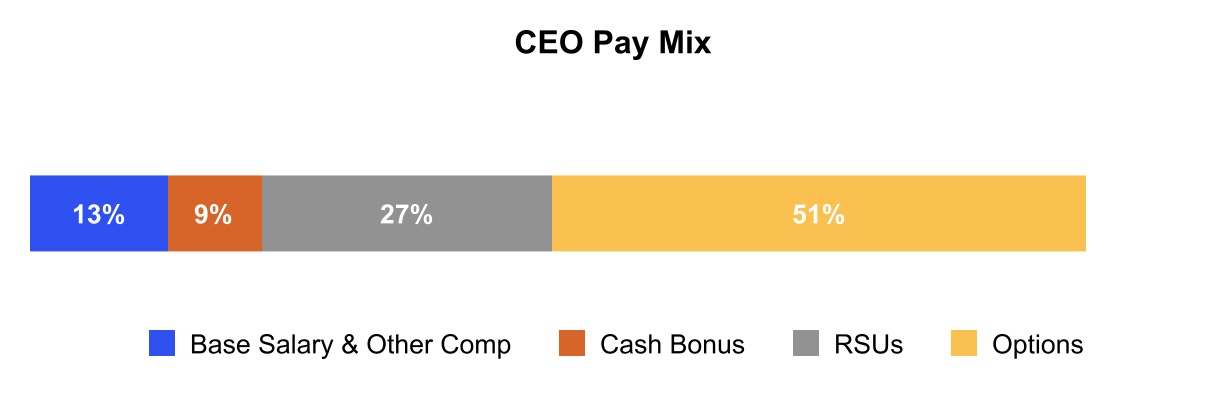
Patrick E.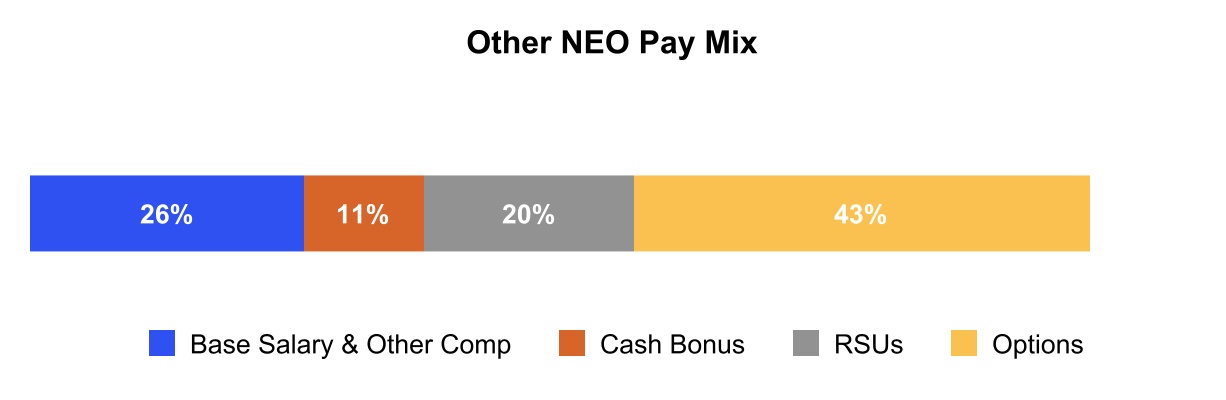
The equity awards granted to the other NEOs who were employed by the Company for the entirety of 2022, including the CEO, were comprised of a mix of RSUs and options.
Base Salaries
The base salaries of our named executive officers are a modest, but important part of their total compensation package, and are intended to reflect their respective positions, duties and responsibilities.Base salary is a visible and stable fixed component of our compensation program.Base salaries for our named executive officers were initially established by the Compensation Committee and/or board of directors at the time each executive was hired.
In reviewing and adjusting base salaries for 2022, the Compensation Committee considered current market data as well as each named executive officer’s total cash compensation (consisting of base salary and target bonus opportunity) and individual performance.
Subsequent to our IPO in 2020, we began a multi-step process to better align the base salaries of our named executive officers with competitive market practices of comparable public companies. In connection with this approach and based on the performance of the company and our named executive officers in 2021, we increased the base salaries of our continuing named executive officers by approximately 3.8% to 14.5% during 2022.
The largest increases were to our CEO and to our CFO, both of whom remained below the median of our peer group.
The following table sets forth the base salaries of our named executive officers for fiscal 2022:
| | | | | | | | | | | | | | | | | | | | |
| Named Executive Officer | | 2021 Base Salary | | 2022 Base Salary | | Year-over-Year % Increase |
| Todd Franklin Watanabe (1) | | $540,000 | | $600,000 | | 11.1% |
| Masaru Matsuda, J.D. (2) | | — | | $415,000 | | —% |
| Patrick Burnett, M.D., Ph.D. (1) | | $443,000 | | $465,000 | | 5.0% |
| Kenneth Lock (1) | | $380,000 | | $395,000 | | 3.9% |
| Scott Burrows (1) | | $345,000 | | $395,000 | | 14.5% |
(1)Salary increases for Mr. Watanabe, Mr. Burnett, M.D., Ph.D. has servedMr. Burrows, and Mr. Lock went into effect on March 1, 2022.
(2)Mr. Matsuda joined the Company in January 2022.
Cash-Based Incentive Compensation
The Company maintains a cash annual incentive compensation program, whereby our named executive officers are eligible to receive cash incentive bonuses payable upon the achievement of specified annual corporate performance goals.
Annual Corporate Performance Goals
The Compensation Committee works with the executive team to develop goals with respect to the Company’s annual incentive compensation program and ultimately recommends a list of goals to the board for approval. The board reviews the strategic, operational and financial components of the goals and approves the goals as well as a weighting for each goal based on its relative importance. Our Chief Executive Officer and executive team provide updates to the board through the course of the year on performance towards these goals. At the end of the year, our Chief Executive Officer presents the Compensation Committee with a proposed score based on the Company’s performance against the goals. After discussion and review, the Compensation Committee recommends to the board for approval the overall corporate achievement score compared to the annual performance goals. This score is then used to establish the annual bonus payments for each named executive officer.
Annual Bonus Process
The Compensation Committee considers input from the Chief Executive Officer when reviewing the annual performance and compensation reviews for our executive officers, and considers input from the non-employee members of our board relative to the performance and compensation of our Chief Executive Officer. The Compensation Committee reviews base salary, annual bonus and equity-based compensation annually as part of this review. The review is typically conducted over a series of meetings beginning at the end of the year and as part of the Company’s broader annual performance review process. The annual corporate score which is determined as described above in “Annual Corporate Performance Goals” is used to determine the size of the Company-wide bonus pool. Our Chief Executive Officer and named executive officers receive a bonus based entirely on corporate performance since they have ultimate operational responsibility for corporate performance. The Compensation Committee retains flexibility to increase or decrease any and all compensation components to reflect performance.
2022 Bonus Targets
We consider annual cash incentive bonuses to be an important component of our total compensation program that provide incentives necessary to retain executive officers. Each named executive officer is eligible to receive an annual performance-based cash bonus based on a specified target annual bonus award amount, expressed as a percentage of the named executive officer’s base salary. Bonus targets are reviewed annually by the Compensation Committee, taking into consideration competitive market data, and adjusted if deemed appropriate by the Compensation Committee. In fiscal 2022, our named executive officers participated in our annual cash incentive bonus program at the following target percentages of base salary:
| | | | | | | | |
| Named Executive Officer | | 2022 Bonus Target |
| Todd Franklin Watanabe | | 60% |
| Masaru Matsuda, J.D. | | 40% |
| Patrick Burnett, M.D., Ph.D. | | 40% |
| Kenneth Lock | | 40% |
| Scott Burrows | | 40% |
2022 Corporate Goal Development and Weighting
We provide our executive officers the opportunity to receive annual cash incentives that are intended to encourage the achievement of corporate performance objectives. In January 2022, the Compensation Committee set the performance objectives for fiscal 2022, which mainly fell into five categories: (i) launch roflumilast (ARQ-151) for plaque psoriasis, (ii) progress roflumilast follow-on indications, (iii) advance the pipeline, (iv) finance and business development, and (v) build and scale corporate infrastructure. Our Corporate Bonus Plan provides the opportunity to achieve the maximum potential bonus of 150% overall if pre-specified stretch objectives are achieved.The charts below summarize our performance objectives, weightings and attainment under our Corporate Bonus Plan for fiscal 2022.
Corporate Goals and Objectives: | | | | | | | | | | | | | | |
| Goal Area and Target Weight | | Objective | | Results Achieved |
Launch Roflumilast Cream for Plaque Psoriasis (50%) | | •FDA approval in plaque psoriasis •10%: Secure ARQ-151 NDA approval by July 31 •Commercial and operational execution | | 40.5% |
|
Progress Roflumilast Follow-on Indications (20%) | | •Completion of key clinical materials •Continuous supply of clinical materials | | 35% |
|
Advance the Pipeline (10%) | | •Progress on early pipeline programs
| | 15% |
| Finance & Business Development (10%) | | •Raise sufficient funds across financing alternatives •Manage cash within a range of budget | | 15% |
|
Build & Scale Corporate Infrastructure (10%) | | •Information technology solutions •Environmental, Social and Governance (ESG) | | 9.5% |
| Total Goals Score: | | 115% |
Launch Roflumilast for Plaque Psoriasis
FDA Approval in Plaque Psoriasis
Gained FDA approval on July 29, 2022 for the treatment of plaque psoriasis in adolescent and adult patients.
Commercial and Operational Execution
Upon approval, it was important for us to get product out into the market quickly so that plaque psoriasis patients in need could get access to product.We were able to get commercial product in channel within 7 business days of approval and shipped approximately 16,000 units of product through year end 2022, slightly under target.Net revenue of $3.1 million in 2022.Achieved coverage with one major payor in 2022 and instituted a patient assistance program, which provides Zoryve® (roflumilast) cream 3% a no cost for financially eligible patients who are uninsured or underinsured.
Progress on Roflumilast Follow-on Indications
Completion of Key Clinical Trials
We delivered on our roflumilast clinical development program as follows:
Roflumilast cream formulation:
On November 15, 2022 and December 12, 2022, we announced positive topline data for roflumilast cream 0.15% for each of the two phase 3 trials in atopic dermatitis (AD) in adults and children 6 years and older, demonstrating significant improvement in signs and symptoms of AD at 4 weeks, including itch, with favorable safety and tolerability.
On December 19, 2022, we announced the submission of a supplemental NDA to the FDA to expand the indication of roflumilast cream 0.3% for the treatment of plaque psoriasis in children down to 2 years of age.
Roflumilast foam formulation:
We have also advanced the development of our roflumilast foam 0.3% formulation, with phase 3 results in two separate indications that should provide the basis for NDA and sNDA submissions for this product.
On June 6, 2022, we announced positive topline data on our roflumilast foam phase 3 trial for the treatment seborrheic dermatitis in adults and adolescents, demonstrating substantial improvement in the most important signs and symptoms of seborrheic dermatitis, including itch, with favorable tolerability and safety.
On September 26, 2022, we announced positive topline data on our roflumilast foam phase 3 trial in scalp and body psoriasis in adults and adolescents, demonstrating strong efficacy, as well as favorable safety and tolerability in the treatment of signs and symptoms of plaque psoriasis in all of these body areas.
Continuous Supply of Clinical Materials
In addition to meeting all commercial launch supply needs, we were able to continue delivering on all supply needs for the Company’s clinical development program in 2022.
Advance the Pipeline
Progress on Early Pipeline Programs
We advanced the Arcutis pipeline. We submitted the IND for ARQ-255, our topical JAK inhibitor therapy specifically designed as a potential treatment for alopecia areata. On December 5, we announced the enrollment of the first subject in our first-in-human Phase 1b study evaluating the safety, tolerability, and PK of ARQ-255 in healthy adult volunteers and individuals with patchy alopecia areata.
In addition, we also formulated a new product candidate for IND-enabling studies.
Further, we completed the acquisition of Ducentis Biotherapeutics Ltd. in September 2022 which brought to us a pre-clinical asset, now ARQ-234, a fusion protein that is a highly selective and potent checkpoint agonist.This offers a potential best-in-class profile in moderate-to-severe atopic dermatitis, and is highly complementary to roflumilast cream in that potential indication.
Finance & Business Development
Raise Funds Across Financing Alternatives
During 2022, we raised over $300 million to fund our commercial launch and pipeline activities.This included over $185 million in equity financing, primarily from a successful $172 million follow-on financing after the approval of ZORYVE. We also received $125 million from Tranche B of our credit facility, which was contingent on U.S. regulatory approval of roflumilast in plaque psoriasis.
Manage Cash Within a Range of Budget
We have implemented rigorous financial planning and reporting processes to ensure that our expenditures are in-line with the budget and priorities of the Company as approved by the Board of Directors.
Build and Scale Corporate Infrastructure
Information Technology Solutions
Innovation is in our blood.We developed Information Technology Solutions for (i) our commercial launch of roflumilast, through the build of a commercial data and analytics solution that provides visibility into our launch trajectory and patient demand metrics, and (ii) our clinical trial program, through the development of a set of data driven tools to accelerate our clinical patient recruitment effort.
Environmental, Social and Governance (ESG)
In 2022, consistent with our ESG Principles, we built and scaled our processes and systems as we evolved from a development to an early commercial organization.The following evidence how we built and scaled:
•We developed a Commercial Quality Management System (QMS) - the required regulatory infrastructure for monitoring the quality of our commercial product.
•We scaled and evolved the processes and policies underlying our corporate healthcare compliance program.
•We conducted and improved year over year on the score of our Arcutis Operating Principles survey with an employee participation rate of nearly 95%.This survey provides data to maintain our company culture, our high level of performance, and staff retention as the Company continues to grow.
Evaluation of Performance Against 2022 Corporate Goals
In January 2023, the board evaluated the Company’s performance against the above goals and determined that the Company had achieved corporate goals for fiscal 2022 at 115% of target levels.
In consideration of the foregoing, in March 2023, pursuant to the terms and conditions of the applicable annual bonus guidelines, the Board approved annual cash bonus payments for each named executive officer as reflected in the table below.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Named Executive Officer | | 2022
Bonus Target | | Corporate Goal
Score Modifier | | 2022 Actual Bonus
Paid as % of Salary | | 2022 Actual
Bonus Paid (1) |
| Todd Franklin Watanabe | | 60% | | 115% | | 69% | | $407,100 |
| Masaru Matsuda, J.D. | | 40% | | 115% | | 46% | | $190,901 |
| Patrick Burnett, M.D., Ph.D. | | 40% | | 115% | | 46% | | $210,297 |
| Kenneth Lock | | 40% | | 115% | | 46% | | $180,550 |
| Scott Burrows | | 40% | | 115% | | 46% | | $177,867 |
(1)2022 bonuses were paid in March 2023 and were calculated by multiplying each NEO’s actual base salary earned in 2022 by their 2022 bonus target, and multiplying the resulting value by the corporate goal score modifier.
2023 Annual Incentive Compensation Program
In March 2023, the Board also approved an annual incentive compensation program for 2023 for our NEOs on substantially similar terms and conditions as our Chief Medical Officer since August 2020. Prior2022 annual incentive compensation program described above and with slightly increased 2023 bonus targets..
Equity-Based Incentive Compensation
We view equity-based compensation as a critical component of our balanced total compensation program. Equity-based compensation creates an ownership culture among our employees that provides an incentive to contribute to the continued growth and development of our business and aligns the interests of executives with those of our stockholders. In evaluating the mix of equity awards for 2022, the Compensation Committee considered market trends as well as practices of our peer group companies, and determined that Dr. Burnett wasa mix of stock options, restricted stock units and where appropriate, performance-based options, would be the Chief Medical Officer at Verrica Pharmaceuticals since April 2018. Priormost appropriate incentive structure for our named executive officers to reward performance over time and achieve our retention objectives. We do not currently have any formal policy for determining the number of equity-based awards to grant to named executive officers.
Stock Options
The Compensation Committee and Board grant stock options to emphasize retention and align the named executive officers’ interests with those of stockholders. These awards provide a necessary balance between performance-based and time-vesting awards. Stock option awards will generally be fully vested four years after the option grant date, subject to continuous service.
Restricted Stock Units
Restricted stock unit (RSUs) are an important retention vehicle for named executive officers, as well as a variable and at-risk component of executive compensation. Market trends reflect their favored use among our peer group and other companies in the biotechnology sector. RSU awards will generally be fully vested four years after the RSU grant date, subject to continuous service.
Performance-Based Equity
In certain cases, the Compensation Committee and/or the Board grant performance-based stock options or performance-based restricted stock units (PSUs) that Dr. Burnett was at Sun Pharmaceuticals where he was Associate Vice Presidentbegin vesting upon the completion of Clinical Development from September 2015specific performance criteria and generally vest over the following four years, subject to March 2018,continuous service. Performance-based grants align the named executive officers’ interests with oversightthose of stockholders.
In connection with Mr. Matsuda commencing services as our General Counsel and Corporate Secretary in January 2022, our Compensation Committee and Board granted him 39,000 PSUs. The PSUs were to become earned based on the achievement of specified performance targets: (i) 19,500 PSUs were to become earned upon approval by the Audit Committee of the dermatologyCompany’s commercial launch compliance program and rheumatology pipeline. Prior(ii) 19,500 PSUs were to Sun Pharmaceuticals, Dr. Burnettbecome earned upon the execution of the first key payor contract with a top plan. The first performance target was at Novartis from 2010achieved on May 27, 2022. The second performance target was achieved on November 18, 2022. Upon the date of achievement of each respective performance target, the first 25% of each applicable grant vested and the remaining portion of each grant will vest in equal 25% installments on each subsequent one year anniversary for the remaining three-year period, subject to August 2015, most recently as Global Program Medical Director. He is a board certified dermatologistMr. Matsuda’s continuous service to the Company through each vesting date.
Equity Grants
The following table sets forth the equity awards granted to our named executive officers in the 2022 fiscal year.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Named Executive Officer | | Grant Date | | Type of Grant | | Stock Options (#) | | RSUs (#) | | Performance RSUs (#) |
| Todd Franklin Watanabe | | March 3, 2022 | | Annual | | 188,100 | | 70,900 | | |
Masaru Matsuda, J.D. (1) | | January 3, 2022 | | New-Hire | | 185,000 | | | | |
| January 3, 2022(2) | | Performance | | | | | | 19,500 |
| January 3, 2022(3) | | Performance | | | | | | 19,500 |
| March 3, 2022 | | Annual | | 35,300 | | 13,300 | | |
| Patrick Burnett, M.D., Ph.D. | | March 3, 2022 | | Annual | | 47,100 | | 17,800 | | |
| Kenneth Lock | | March 3, 2022 | | Annual | | 35,300 | | 13,300 | | |
| Scott Burrows | | March 3, 2022 | | Annual | | 35,300 | | 13,300 | | |
(1)Mr. Matsuda joined the Company in January 2022 and was a memberawarded new-hire stock options and performance-based stock awards. Mr. Matsuda’s PSU’s were subject to the achievement of certain milestones.
(2)The milestone associated with this performance-based stock award was achieved on May 27, 2022, at which time 25% of the medical facultyapplicable grant vested. The remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period.
(3)The milestone associated with this performance-based stock award was achieved on November 18, 2022, at Vanderbilt University which time 25% of the applicable grant vested. The remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period.
Our employee benefit programs are designed to provide a competitive level of benefits to all of our full-time employees, including our named executive officers.All full-time employees are eligible to participate in our health and welfare plans, including:
•Medical, Centerdental, and vision benefits;
•Medical and dependent care flexible spending accounts;
•401(k) retirement savings plan, pursuant to which we make matching safe-harbor contributions under our 401(k) plan at 100% of the first 3% of each participant’s contributions, plus 50% of each participant’s next 2% of contributions, for a maximum aggregate matching contribution of 4%;
•Long-term disability insurance; and
•Life insurance.
We believe the benefits described above are necessary and appropriate to provide a competitive compensation package to all of our full-time employees.
Perquisites and Other Personal Benefits
Currently, we do not view perquisites or other personal benefits as a significant component of our executive compensation program. Accordingly, we do not generally provide perquisites or other personal benefits to our executive officers, including our named executive officers, except as generally made available to our employees, or in situations where we believe it is appropriate to assist an Assistant Professorindividual in the performance of Dermatologyhis or her duties, to make our executive officers more efficient and effective and for recruitment and retention purposes.
In connection with travel to and from 2004our corporate headquarters, we have reimbursed the following named executive officers for travel and housing expenses as follows:
| | | | | | | | |
| Named Executive Officer | | Aggregate payments in 2022 |
| Todd Franklin Watanabe | | $4,752 |
| Masaru Matsuda, J.D. | | $8,117 |
| Patrick Burnett, M.D., Ph.D. | | $10,290 |
| Kenneth Lock | | $15,366 |
| Scott Burrows | | — |
In the future, we may provide perquisites or other personal benefits in limited circumstances, such as those described in the preceding paragraph. All future practices with respect to 2010. Dr. Burnett holds an M.D.perquisites or other personal benefits will be approved and Ph.D.subject to periodic review by the Compensation Committee.
Employment and Severance Arrangements
We have entered into employment agreements with all of our named executive officers, which provide for base salaries, target cash incentives, and benefit plan participation, as well as certain additional benefits as described below. We entered into a new employment agreement with Mr. Matsuda in neuroscience from Johns Hopkins School of Medicineconnection with him joining the Company in January 2022 and have described such agreement in detail below.
Todd Franklin Watanabe
We entered into a B.S.continued employment agreement with Mr. Watanabe, our President and Chief Executive Officer, in BiologyJanuary 2020 (the “Watanabe Continued Employment Agreement”). The Watanabe Continued Employment Agreement outlined his initial base salary and Biochemistry from the University of Iowa.
Kenneth A. Lock has servedtarget bonus opportunity, as our Chief Commercial Officer since October 2019. Prior to joining Arcutis, he servedwell as the Executive Directoropportunity to continue participating in our employee benefit plans. In a November 2020 First Amendment to Continued Employment Letter, we amended the agreement to indicate that Mr. Watanabe’s Principal Place of SalesEmployment is now in Utah and Marketing at Gilead Sciences, concurrently leadingnot California.
Under Mr. Watanabe’s severance and change in control agreement (the “Watanabe Severance and Change in Control Agreement”) that went into effect on the Inflammation and Pulmonary Hypertension U.S. commercial franchises from December 2013date of our initial public offering, he is entitled to August 2019. Prior to Gilead, Mr. Lock was employed at Amgen, Inc. from March 2007 to November 2013, where he was involvedcertain benefits in the prelaunch global developmentevent of Repathaa termination of employment with cause, or resignation for hyperlipidemiagood reason (both during and also held U.S. brand marketingoutside a change in control).
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Mr. Watanabe will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the qualifying termination for twelve (12) months following termination; and sales leadership roles
•Subject to Mr. Watanabe’s timely election for Enbrelcontinued coverage under COBRA, we shall pay, or reimburse, his monthly premium for Rheumatoid Arthritishim and Psoriasis. From June 2003his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Mr. Watanabe with a monthly cash payment equal to February 2007the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Mr. Lock was at Wyeth Pharmaceuticals whereWatanabe will be entitled to receive the following benefits:
•Severance pay in an amount equal to eighteen (18) months of his base salary rate in effect immediately prior to the qualifying termination plus 1.5 times his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over an eighteen-month period;
•Subject to Mr. Watanabe’s timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) eighteen (18) months, (b) the date when he held various positions including Strategic Planning, International Commercial Operations,receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Mr. Watanabe with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings; and Marketing for Enbrel in both Rheumatology
•Full acceleration and Dermatology. He started his career in process developmentimmediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions, as well as the performance-based stock options granted on March 13, 2019.
The amounts set forth above are subject to Mr. Watanabe’s timely delivery to us of a general release of claims that becomes effective and biologics manufacturing at IDEC Pharmaceuticals in 1996.irrevocable.
Masaru Matsuda, J.D.
We entered into an executive employment agreement with Mr. Lock received both his B.S. in Biochemistry / Cell Biology and B.A. in Psychology from University of California, San Diego and completed his M.B.A at Cornell University.
Patricia A. Turney has served asMatsuda, our Senior Vice President and General Counsel, in December 2021 (the “Matsuda Employment Agreement”) in connection with him joining us in January 2022. The Matsuda Employment Agreement outlined his initial base salary and target bonus opportunity, as well as the opportunity to participate in our employee benefit plans. In addition, the Matsuda Employment Agreement provides for reimbursement by us for travel expenses incurred for travel to and from, and housing, at our corporate headquarters, in accordance with the Company’s expense reimbursement policy, as in effect from time to time.
Under Mr. Matsuda’s severance and change in control agreement (the “Matsuda Severance and Change in Control Agreement”) that went into effect in January 2022, he is entitled to certain benefits in the event of Manufacturing since November 2019. Priora termination of employment with cause, or resignation for good reason (both during and outside a change in control).
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Mr. Matsuda will be entitled to joining Arcutis, she wasreceive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the qualifying termination for nine (9) months following termination; and
•Subject to Mr. Matsuda’s timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) nine (9) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Mr. Matsuda with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Mr. Matsuda will be entitled to receive the following benefits:
•Severance pay in an amount equal to twelve (12) months of his base salary rate in effect immediately prior to the qualifying termination plus his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over an twelve-month period;
•Subject to Mr. Matsuda’s timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Mr. Matsuda with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings; and
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions, as well as the performance-based stock options granted on January 3, 2022.
The amounts set forth above are subject to Mr. Matsuda’s timely delivery to us of a general release of claims that becomes effective and irrevocable.
Patrick Burnett, M.D., Ph.D.
We entered into an executive employment agreement with Dr. Burnett, our Senior Vice President External Supplyand Chief Medical Officer, in July 2020 (the “Burnett Employment Agreement”) in connection with him joining us in August 2020. The Burnett Employment Agreement outlined his initial base salary and target bonus opportunity, as well as the opportunity to participate in our employee benefit plans. In addition, the Burnett Employment Agreement provides for Amgen, Inc., where she was responsiblereimbursement by us for travel expenses incurred for travel to and from, and housing, at our corporate headquarters, up to a maximum of $5,000 per month.
Under Dr. Burnett’s severance and change in control agreement (the “Burnett Severance and Change in Control Agreement”) that went into effect in July 2020, he is entitled to certain benefits in the event of a termination of employment with cause, or resignation for good reason (both during and outside a change in control).
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Dr. Burnett will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the qualifying termination for nine (9) months following termination; and
•Subject to Dr. Burnett’s timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) nine (9) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Dr. Burnett with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Dr. Burnett will be entitled to receive the following benefits:
•Severance pay in an amount equal to twelve (12) months of his base salary rate in effect immediately prior to the qualifying termination plus his annual bonus amount for the manufacturethen-current fiscal year based on 100% of target performance, paid out in substantially equal installments over $5Ban twelve-month period;
•Subject to Dr. Burnett’s timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in annual product sales, more than 250 external suppliers,lieu of paying or reimbursing the premiums, we shall instead provide Dr. Burnett with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings; and 55 contract manufacturing sites spanning 10 countries. Previously, she led Amgen’s Manufacturing Site Operations in
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions, as well as the performance-based stock options granted on August 3, 2020.
The Netherlands, supplying patients in over 75 countries. Ms. Turney servedamounts set forth above are subject to Dr. Burnett’s timely delivery to us of a general release of claims that becomes effective and irrevocable.
Kenneth Lock
We entered into a continued employment agreement with Amgen for more than 23 years,Mr. Lock, our Senior Vice President and held a wide variety of leadership roles with increasing responsibility within Manufacturing, Engineering, EH&S, R&D, and Quality. She received her B.S. in Mathematics and Engineering from the US Naval Academy, and her M.B.A. from UCLA’s Anderson School of Management. Prior to her career at Amgen, Ms. Turney was a U.S. Naval Aviator and served in the US Navy in various locations around the world.
Matthew R. Moore joined Arcutis as Chief BusinessCommercial Officer, in January 2021. Mr. Moore has over 20 years of strategy, transaction2020 (the “Lock Continued Employment Agreement”). The Lock Continued Employment Agreement outlined his initial base salary and operations experience in the biopharmaceutical industry. Most recently, he served as Vice President, Corporate Business Development and Alliance Management at Allergan, where he led worldwide strategy and business development for the company’s $4B+ Medical Aesthetics business unit. During his tenure at Allergan and its predecessor companies, Actavis and Forest Labs, Mr. Moore was responsible for creating and executing business development growth strategies across multiple therapeutic areas including medical aesthetics, neuroscience, anti-infectives and hospital products. In addition, Mr. Moore served as a key deal team member in Actavis’ transformational acquisition of Allergan and Allergan’s ultimate sale to AbbVie. Prior to Allergan, Mr. Moore held executive roles at DOV Pharmaceutical and he started his career in the healthcare investment banking group at CIBC Oppenheimer. Mr. Moore earned his B.A. in Psychology from Trinity College.
EXECUTIVE COMPENSATION
The following discusses our executive compensation program for our 2020 named executive officers (“NEOs”). As an “emerging growth company” as defined in the JOBS Act, we are not required to include a Compensation Discussion and Analysis section and have elected to comply with the scaled disclosure requirements applicable to emerging growth companies. In addition, as an emerging growth company, we are not required to hold an advisory vote to approve the compensation of our NEOs, or “say-on-pay” vote.
Our Compensation Committee, the members of which are appointed by our Board, is responsible for establishing, implementing and monitoring our compensation philosophy and objectives. We seek to ensure that the total compensation paid to our executive officers is reasonable and competitive. Compensation of our executives is structured around the achievement of individual performance and near-term corporate targetstarget bonus opportunity, as well as long-term business objectives.the opportunity to continue participating in our employee benefit plans. In addition, the Lock Continued Employment Agreement provides for reimbursement by us for travel expenses incurred for travel to and from, and housing, at our corporate headquarters, up to a maximum of $4,000 per month..
Our NEOs, whoUnder Mr. Lock’s severance and change in control agreement (the “Lock Severance and Change in Control Agreement”) that went into effect on the date of our initial public offering, he is entitled to certain benefits in the event of a termination of employment with cause, or resignation for good reason (both during and outside a change in control).
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Mr. Lock will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the Qualifying Termination for nine (9) months following termination;
•Subject to Mr. Lock’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) nine (9) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Mr. Lock with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Mr. Lock will be entitled to receive the following benefits:
•Severance pay in an amount equal to twelve (12) months of his base salary rate in effect immediately prior to the qualifying termination plus his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over an twelve-month period;
•Subject to Mr. Lock’s timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Mr. Lock with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings; and
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions.
The amounts set forth above are subject to Mr. Lock’s timely delivery to us of a general release of claims that becomes effective and irrevocable.
Scott Burrows
We entered into an employment agreement with Mr. Burrows, our principalSenior Vice President and Chief Financial Officer, in April 2019 (the “Burrows Employment Agreement”). The Burrows Employment Agreement outlined his initial base salary and target bonus opportunity, as well as the opportunity to participate in our employee benefit plans.
Under Mr. Burrows’ severance and change in control agreement (the “Burrows Severance and Change in Control Agreement”) that went into effect in April 2021 in conjunction with his promotion to Senior Vice President and Chief Financial Officer, he is entitled to certain benefits in the event of a termination of employment with cause, or resignation for good reason (both during and outside a change in control).
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Mr. Burrows will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the Qualifying Termination for nine (9) months following termination;
•Subject to Mr. Burrows’ timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) nine (9) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Mr. Burrows with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Mr. Burrows will be entitled to receive the following benefits:
•Severance pay in an amount equal to twelve (12) months of his base salary rate in effect immediately prior to the qualifying termination plus his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over an twelve-month period;
•Subject to Mr. Burrows’ timely election for continued coverage under COBRA, we shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. We may elect that, in lieu of paying or reimbursing the premiums, we shall instead provide Mr. Burrows with a monthly cash payment equal to the amount we would have otherwise paid for his monthly premium, less applicable tax withholdings; and
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions, as well as the performance-based stock options granted on August 24, 2021.
The amounts set forth above are subject to Mr. Burrows’ timely delivery to us of a general release of claims that becomes effective and irrevocable.
Potential Payments Upon Termination or Change in Control
The amount of compensation and benefits payable to each named executive officer in various termination and change in control situations has been estimated in the tables below. The value of the option and RSU vesting accelerations was calculated for each of the tables below on the assumption that the change in control and executive’s employment termination occurred on December 30, 2022. The closing price of our common stock on December 30, 2022 was $14.80 (the last trading day in 2022), which was used as the value of our common stock in the change in control calculations. The value of the option vesting acceleration was calculated by multiplying the number of unvested option shares subject to vesting acceleration as of December 30, 2022 by the difference between the closing price of our common stock as of December 30, 2022 and the two most highly-compensatedexercise price. No value is attributed to unvested options subject to acceleration which have exercise prices above the closing market price of our common stock on December 30, 2022. The value of RSUs was calculated by multiplying the number of unvested RSUs subject to vesting acceleration as of December 30, 2022 by the closing price of our common stock on December 30, 2022. The respective severance and change in control agreement for each named executive officers (other than our principalofficer includes a modified cutback provision, such that if any severance payments or benefits would constitute a “parachute payment” and would be subject to the excise tax imposed by Section 4999 of the Code, the aggregate benefits will either be delivered in full or delivered in a lesser amount that would result in no portion of the aggregate benefits being subject to the excise tax, whichever results in the receipt by the named executive officer) servingofficer of the greatest amount of aggregate benefits on an after-tax basis. For purposes of the table set forth below, it has been assumed that the payments and benefits will not be reduced pursuant to the preceding sentence and, accordingly, include the full value of such payments and benefits.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Named Executive Officer | | Termination by Company without Cause or resignation for Good Reason not in Change in Control period ($) | | Termination by Company without Cause or resignation for Good Reason during a Change in Control period ($) | | Termination by Company on Death or Disability ($) | | Termination by Company for Cause or resignation without Good Reason ($) |
Todd Franklin Watanabe Base salary continuation (1) Bonus continuation (1) Lump sum bonus payment (2) COBRA premiums (3) Accelerated vesting of equity awards | |
600,000
--
--
25,733
-- | |
900,000
540,000
407,100
38,599
2,968,827 | |
--
--
--
--
-- | |
--
--
--
--
-- |
Masaru Matsuda, J.D. Base salary continuation (1) Bonus continuation (1) Lump sum bonus payment (2) COBRA premiums (3) Accelerated vesting of equity awards | |
311,250
--
--
25,955
-- | |
415,000
166,000
190,901
34,606
629,740 | |
--
--
--
--
-- | |
--
--
--
--
-- |
Patrick Burnett, M.D., Ph.D. Base salary continuation (1) Bonus continuation (1) Lump sum bonus payment (2) COBRA premiums (3) Accelerated vesting of equity awards | |
345,000
--
--
24,436
-- | |
460,000
184,000
210,297
32,581
802,722 | |
--
--
--
--
-- | |
--
--
--
--
-- |
Kenneth Lock Base salary continuation (1) Bonus continuation (1) Lump sum bonus payment (2) COBRA premiums (3) (4) Accelerated vesting of equity awards | |
296,250
--
--
--
-- | |
395,000
158,000
180,550
--
644,720 | |
--
--
--
--
-- | |
--
--
--
--
-- |
Scott Burrows Base salary continuation (1) Bonus continuation (1) Lump sum bonus payment (2) COBRA premiums (3) Accelerated vesting of equity awards | |
296,250
--
--
26,810
-- | |
395,000
158,000
177,867
35,746
372,112 | |
--
--
--
--
-- | |
--
--
--
--
-- |
(1)Reflects the aggregate amount of cash severance payable to each named executive officer based on a multiple of base salary and target annual incentive compensation as of 2022 pursuant to the terms of the respective named executive officer’s severance and change in control agreement, as described above.
(2)The lump sum bonus payment amounts represent the accrued bonus payments for fiscal year 2022 that would have been unpaid to named executive officers as of December 31, 2022.
(3)Represents the estimated value of the continuation of health benefits that each named executive officer would have been entitled to receive upon a termination of employment as of December 31, 2022, as based on actual 2022 premiums and the terms of the respective named executive’s officer’s severance and change in control agreement, as described above.
(4)During 2022, Mr. Lock was not enrolled in the Arcutis medical, dental, and vision health benefits plans.
Accounting and Tax Considerations
As a general matter, our Board of Directors review and consider the various tax and accounting implications of compensation programs we utilize.
Deductibility of Executive Compensation
Generally, Section 162(m) of the Code (“Section 162(m)”) disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any fiscal year to certain specified executive officers. These executive officers include our chief executive officer, chief financial officer, and any employee who is among the three highest compensated executive officers for the taxable year (other than the chief executive officer and chief financial officer), regardless of whether the executive officer is serving at the end of the public company’s taxable year and regardless of whether the executive officer’s compensation is subject to disclosure for the last completed fiscal year under the applicable SEC rules (a “Covered Employee”). In addition, once an individual becomes a Covered Employee for any taxable year beginning after December 31, 2016, that individual will remain a Covered Employee for all future years, including following any termination of employment.
While we may take into account deductibility of compensation when making compensation decisions, our Compensation Committee believes that stockholder interests are best served if our Compensation Committee retains maximum flexibility to design executive compensation programs that meet stated business objectives. Therefore, our Compensation Committee has approved base salaries and other compensation awards for our executive officers that may not be fully deductible because of the deduction limit of Section 162(m) and expects in the future to approve additional compensation that is not deductible for federal income tax purposes.
Accounting for Stock-Based Compensation
We follow the Financial Accounting Standard Board’s Accounting Standards Codification Topic 718 (“FASB ASC Topic 718”) for our stock-based compensation awards. FASB ASC Topic 718 requires us to measure the compensation expense for all share-based payment awards made to our employees and non-employee members of our board of directors, including options to purchase shares of our common stock and other stock awards, based on the grant date “fair value” of these awards. This calculation is performed for accounting purposes and reported in the executive compensation tables required by the federal securities laws, even though the recipient of the awards may never realize any value from their awards.
Other Compensation Policies and Practices
•No Pledging, Hedging or Similar Monetization Transactions
We maintain an Insider Trading Compliance Policy that prohibits our employees, officers and directors from engaging in pledging Company securities, hedging or similar monetization transactions, including put options, call options, short sales and exchange fund transactions. Our policy also prohibits employees, officers and directors from using or pledging securities as collateral in margin accounts or for loans unless approved by our designated compliance officer under the policy.
•Clawback Policy
We maintain a Clawback Policy that allows the Company to seek to recover certain incentive compensation paid to its senior executives in situations where recoverable events have taken place.
•Stock Ownership Guidelines
We apply Stock Ownership Guidelines to senior executives, including NEOs, and non-employee directors to align their long-term interests with those of the Company’s shareholders.
Compensation Risk Assessment
Consistent with the SEC’s disclosure requirements, Pay Governance, our independent compensation consultant, assessed our compensation programs for all employees. We have concluded that our compensation policies and practices do not create risks that are reasonably likely to have a material adverse effect on us.
The Compensation Committee monitors our compensation programs on an annual basis and expects to make modifications as necessary to address any changes in our business or risk profile.
Pay Equity Assessment
In 2022, we retained Aon Human Capital Solutions to conduct a pay equity assessment of our United States employees below the Senior Vice President level. The purpose of this assessment was to identify potential pay gaps by analyzing whether factors like race, ethnicity or gender affect employee pay, while simultaneously controlling for factors that could legitimately influence compensation, such as function, pay grade, manager versus individual contributor, education, span of control, tenure in role, and experience. Aon utilized regression models to conduct the assessment and found no evidence of a systemic pay equity issue across Arcutis.
CEO Pay Ratio Disclosure
Under the Dodd-Frank Act and the related SEC rule (the “Rule”), we are required to provide our stockholders with specified disclosure regarding the relationship of our Chief Executive Officer’s total compensation to the total compensation of our median employee, referred to as “pay-ratio” disclosure. For 2022, the annual total compensation of our Chief Executive Officer, for purposes of this disclosure, was $4,580,742, and the compensation of our median employee was $310,773, resulting in a pay ratio of approximately 15:1. The annual total compensation amounts for our Chief Executive Officer and our median employee include actual base salary earned in 2022, actual bonus earned in 2022 and paid in March 2023, the grant date fair value of the annual equity awards received in 2022, and other compensation received. Due to an 81% increase in our total headcount from 2021 to 2022, and because our 2021 median employee was promoted in 2022, we selected a new median employee for our 2022 CEO Pay Ratio Disclosure.
In accordance with SEC rules, we have identified the median employee as of December 31, 2022 by: (i) aggregating for each applicable employee: (a) annual base salary rate for salaried employees (or annualized rate for hourly employees, excluding overtime), (b) the target incentive pay for fiscal year 2022, and (c) the target value of the annual equity awards, excluding new-hire awards, for fiscal year 2022, and (ii) ranking this compensation measure for our employees from lowest to highest. This
calculation was performed for all employees employed by us as of December 31, 2022, excluding the Chief Executive Officer, whether employed on a full-time, part-time or seasonal basis. In making this determination, we annualized the compensation of employees who were employed by the Company for less than the entire fiscal year. This compensation measure was consistently applied to all employees included in the calculation and reasonably reflects the annual compensation of employees. All amounts paid in currencies other than US Dollars were converted to US Dollars based on the applicable average annual exchange rates. Since the originally identified 2022 median employee was a new hire with anomalous compensation characteristics, we selected as our median employee a continuing employee whose 2022 compensation was substantially similar to that of the original median employee.
The pay ratio reported above is a reasonable estimate calculated in a manner consistent with SEC rules based on our internal records and the methodology described above. The SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. Therefore, the pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios. Mr. Watanabe’s compensation as Chief Executive Officer for 2022 was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Chief Executive Officer | | Year | | Salary ($) | | Bonus ($)(1) | | Stock Awards ($)(2) | | Option Awards ($)(3) | | All Other Compensation ($)(4) | | Total ($) |
| Todd Franklin Watanabe | | 2022 | | $590,000 | | $407,100 | | $1,252,803 | | $2,314,700 | | $16,952 | | $4,581,555 |
(1)Amounts reported in this column for 2022 represent cash annual incentive bonuses approved in March 2023 by our Board for fiscal year 2022, following the achievement of certain corporate goals as determined by the Compensation Committee and as detailed in the heading “Compensation Discussion & Analysis – Evaluation of Performance Against 2022 Corporate Goals”.
(2)The amount in this column reflects the grant aggregate fair value of RSUs as of the grant date.
(3)The amount in this column reflects the grant date aggregate fair value of stock options, using the Black-Scholes value as of the grant date.
(4)Amount includes $12,200 for matching contributions under our 401(k) plan and $4,752 for reimbursements for travel and housing related to travel to Corporate headquarters.
Pay Versus Performance Table
The following table sets forth information regarding the total compensation, for services rendered in all capacities, that was paid to, awarded to or earned by our CEO (referred to below as our “PEO” or princial executive officer) (and on average to our non-CEO NEOs, or our “Other NEOs”), as compared to compensation actually paid (“CAP”) to our CEO (and on average to our Other NEOs) and certain Company and peer performance measures during the years ended December 31, 2022, 2021 and 2020, were:as calculated in accordance with Item 402(v) of Regulation S-K (the “Pay Versus Performance Table”). Please note that we have not included a company selected financial performance measure in the table below as none of our NEOs’ compensation in 2022 was based on any financial performance measures (and were instead based on certain non-financial performance measures, including those related to our research and development).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | Value of Initial Fixed $100 Investment Based on: | | |
| Year | | Summary Compensation Table Total for PEO ($) | | Compensation Actually Paid to PEO ($)(1) | | Average Summary Compensation Table Total for Other NEOs ($) | | Average Compensation Actually Paid to Other NEOs ($)(1) | | Total Shareholder Return ($) | | Peer Group Total Shareholder Return ($)(2) | | Net Income ($) |
| 2022 | | $4,581,555 | | $2,422,054 | | $2,310,749 | | $1,326,466 | | $67.89 | | $117.87 | | $(311,458,000) |
| 2021 | | $4,284,123 | | $441,904 | | $3,070,296 | | $1,021,432 | | $95.14 | | $132.30 | | $(206,356,000) |
| 2020 | | $2,750,883 | | $16,770,089 | | $4,058,365 | | $5,681,144 | | $129.04 | | $133.14 | | $(135,678,000) |
(1)Amounts represent compensation actually paid to our CEO (which was Todd Franklin Watanabe Presidenteach year) and Chiefthe average compensation actually paid to our Other NEOs for the relevant fiscal year, as determined under SEC rules (and described below), which includes:
| | | | | | | | | |
| Year | | Other NEOs | |
| 2022 | | Masaru Matsuda, Patrick Burnett, Kenneth Lock and Scott Burrows | |
| 2021 | | Matthew Moore, Scott Burrows, Patrick Burnett, David Osborne and John Smither | |
| 2020 | | Patrick Burnett and Kenneth Lock | |
CAP to our NEOs represents the “Total” compensation reported in the Summary Compensation Table for the applicable fiscal year, as adjusted as follows:
| | | | | | | | | | | | | | | | | | | | |
| 2022 | 2021 | 2020 |
| Adjustments | PEO | Average Other NEOs | PEO | Average Other NEOs | PEO | Average Other NEOs |
| Deduction for Amounts Reported under the “Stock Awards” and “Option Awards” Columns in the Summary Compensation Table for Applicable FY | $(3,567,503) | $(1,687,369) | $(3,343,926) | $(2,577,658) | $(2,016,253) | $(3,610,916) |
| Increase based on ASC 718 Fair Value of Awards Granted during Applicable FY that Remain Unvested as of Applicable FY End, determined as of Applicable FY End | $2,521,704 | $1,042,588 | $1,708,488 | $847,741 | $1,764,575 | $3,814,469 |
| Increase based on ASC 718 Fair Value of Awards Granted during Applicable FY that Vested during Applicable FY, determined as of Vesting Date | $467,924 | $143,171 | $290,507 | $240,204 | $232,084 | $48,201 |
| Increase/deduction for Awards Granted during Prior FY that were Outstanding and Unvested as of Applicable FY End, determined based on change in ASC 718 Fair Value from Prior FY End to Applicable FY End | $(1,295,222) | $(418,767) | $(2,221,420) | $(458,958) | $9,419,583 | $1,052,321 |
| Increase/deduction for Awards Granted during Prior FY that Vested During Applicable FY, determined based on change in ASC 718 Fair Value from Prior FY End to Vesting Date | $(286,404) | $(63,906) | $(275,868) | $(100,193) | $4,619,217 | $318,704 |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| TOTAL ADJUSTMENTS | $(2,159,501) | $(984,283) | $(3,842,219) | $(2,048,864) | $14,019,206 | $1,622,779 |
(2)For the relevant fiscal year, represents the cumulative TSR (the “Peer Group TSR”) of the Nasdaq Biotechnology Index (the “Peer Group”), which we also utilize in the stock performance graph required by Item 201(e) of Regulation S-K included in our Annual Report for the year ended December 31, 2022. The comparison assumes $100 was invested for the period starting January 31, 2020 (Date of Arcutis Biotherapeutics IPO) through the end of the listed year in the Company and in the Peer Group, respectively. Historical stock performance is not necessarily indicative of future stock performance.
Narrative Disclosure to Pay Versus Performance Table
Description of Between CEO and Other CEO CAP and Financial Performance Measures
The graphs below compare the CAP to our CEO and the average of the CAP to our Other NEOs, with (i) our cumulative TSR, (ii) our Peer Group TSR, and (iii) our net income, in each case, for the fiscal years ended December 31, 2020, 2021 and 2022. CAP is influenced by numerous factors including, but not limited to, the timing of new grant issuances and award vesting, NEO mix, share price volatility during the fiscal year, our mix of performance metrics and other factors.
TSR amounts reported in the graph assume an initial fixed investment of $100 was invested for the period starting December 31, 2019, through the end of the listed year in the Company and our Peer Group.
Although Item 402(v) of Regulation S-K requires the description of the relationship between the compensation actually paid for our NEOs and our net income, we do not currently utilize GAAP or non-GAAP net income as a performance measure in any of our incentive programs. As a result, the impact of year-over-year fluctuations in our net income has less of an impact on compensation actually paid. The key factor that drove the changes in compensation actually paid is the fluctuations of our stock price.
Pay Versus Performance Tabular List
In the years covered by this table, we did not use financial performance measures for our short of long-term incentive compensation for our NEOs. As described in more detail in “Executive Compensation – Compensation Discussion and Analysis,” our executive compensation program reflects a variable “pay-for-performance” philosophy. Over the three years since our initial public offering, we have not used financial or other performance measures to align executive compensation with our performance. Therefore, this analysis only covers the performance measures presented in the Pay-Versus-Performance Table. Moreover, while we generally seek to prioritize long-term performance as our primary incentive for our CEO and our other NEOs, we do not specifically align our performance measures with compensation that is actually paid (as computed in accordance with Item 402(v) of Regulation S-K) for a particular year.
Advisory Vote on Executive Officer;Compensation (“Say-on-Pay” vote)
•Patrick E. Burnett, M.D.,As described above, in 2022 we held our first non-binding stockholder advisory vote on the compensation of our named executive officers, and on the frequency of any future Say-on-Pay votes. In setting the form and amount of compensation for our named executive officers, the Compensation Committee considered the voting results from this non-binding advisory Say-On-Pay vote as well as feedback received from stockholders throughout the year. Stockholder support for our fiscal 2021 compensation program was strong (approximately 95% support at our 2022 Annual Meeting of Stockholders) and did not lead us to make any fundamental changes to our executive compensation program. We will be conducting our next Say-on-Pay vote on the compensation of our named executive officers at this upcoming 2023 Annual Meeting of Stockholders as a result of our non-binding Say-on-Frequency vote from the 2022 Annual Meeting of the Stockholders. See the section titled “Proposal No. 3 Approval, on a Non-Binding, Advisory Basis, of the Compensation of our Named Executive Officers” for more information. We value the opinions of our stockholders and will consider the outcome of future Say-on-Pay votes, as well as any feedback received throughout the year, when making compensation decisions for our executive officers.
Compensation Committee Report
This report of the Compensation Committee shall not be deemed to be incorporated by reference into any filing made by Arcutis Biotherapeutics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, notwithstanding any general statement contained in any such filing incorporating this proxy statement by reference, except to the extent the company incorporates such report by specific reference.
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis with management. Based on this review and these discussions, we have recommended to the Board of Directors that the Compensation Discussion and Analysis be included in the Annual Report on Form 10-K and the proxy statement of Arcutis Biotherapeutics, Inc.
The preceding report has been furnished by the following members of the Compensation Committee:
Bhaskar Chaudhuri Ph.D., Chief Medical Officer; andChair
•Kenneth A. Lock, Chief Commercial Officer.Patrick Heron
Keith Leonard
2023 Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and Principal Position | | Year | | Salary ($) | | Stock Awards ($) (1) | | Option Awards ($) (1) | | Non-Equity Incentive Plan Compensation ($) (2) | | All Other Compensation ($) (3) | | Total ($) |
| Todd Franklin Watanabe | | | | | | | | | | | | | | |
| President and Chief | | 2020 | | 441,667 | | 800,690 | | 1,215,563 | | 281,563 | | 11,400 | | 2,750,883 |
| Executive Officer | | 2019 | | 390,000 | | — | | 580,546 | | 169,750 | | — | | 1,140,296 |
| Patrick E. Burnett | | 2020 | | 170,833 | | 867,315 | | 5,601,100 | | 209,100 | | 696 | | 6,849,044 |
| Chief Medical Officer | | | | | | | | | | | | | | |
| Kenneth A. Lock | | 2020 | | 335,000 | | 248,490 | | 504,926 | | 170,850 | | 8,426 | | 1,267,692 |
| Chief Commercial | | 2019 | | 66,932 | | — | | 674,797 | | 24,346 | | — | | 766,075 |
| Officer | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and Principal Position | | Year | | Salary ($) | | Stock Awards ($) (1) | | Option Awards ($) (1) | | Non-Equity Incentive Plan Compensation ($) (2) | | All Other Compensation ($) (3) | | Total ($) |
| Todd Franklin Watanabe | | 2022 | | 590,000 | | 1,252,803 | | 2,314,700 | | 407,100 | | 16,952 | | 4,581,555 |
| President and Chief | | 2021 | | 525,000 | | 1,060,788 | | 2,283,138 | | 393,750 | | 21,447 | | 4,284,123 |
| Executive Officer | | 2020 | | 441,667 | | 800,690 | | 1,215,563 | | 281,563 | | 11,400 | | 2,750,883 |
| Masaru Matsuda, J.D. (4) | | 2022 | | 415,000 | | 1,123,821 | (5) | 3,392,725 | | 190,901 | | 20,317 | | 5,142,764 |
| General Counsel | | | | | | | | | | | | | | |
| Patrick E. Burnett, | | 2022 | | 457,167 | | 314,526 | | 579,598 | | 210,297 | | 22,490 | | 1,584,078 |
| M.D., Ph.D. | | 2021 | | 437,500 | | 308,180 | | 662,847 | | 218,750 | | 43,851 | | 1,671,128 |
| Chief Medical Officer | | 2020 | | 170,833 | | 867,315 | (6) | 5,601,100 | | 209,100 | | 696 | | 6,849,038 |
| Kenneth Lock | | 2022 | | 392,500 | | 235,011 | | 434,391 | | 180,550 | | 27,566 | | 1,270,018 |
| Chief Commercial | | 2021 | | 373,333 | | 308,180 | | 662,847 | | 186,667 | | 33,354 | | 1,564,381 |
| Officer | | 2020 | | 335,000 | | 248,490 | | 504,926 | | 170,850 | | 8,426 | | 1,267,692 |
Scott Burrows (7) | | 2022 | | 386,667 | | 235,011 | | 434,391 | | 177,867 | | 12,200 | | 1,246,136 |
| Chief Financial Officer | | 2021 | | 330,750 | | 90,832 | | 1,481,820 | (8) | 165,375 | | 11,600 | | 2,080,377 |
(1)Amounts reflect the full grant date fair value of stock and option awards computed in accordance with ASC Topic 718. See Note 910 of the audited financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 20202022 for the assumptions used in calculating these amounts. These amounts do not correspond to the actual value that may be recognized by the NEOs upon vesting of the applicable awards.
(2)Amounts represent the annual performance-based cash incentive earned by our NEOs based on the achievement of certain corporate performance objectives and individual performance during 2020.2022. These amounts were paid to the NEOs in March 2021.2023. Please see the descriptions of the annual performance incentive payments paid to our NEOs under “2020“Cash-Based Incentive Compensation” below. Trackabove.
(3)Amount represents Companyour matching contributions under our 401(k) plan.plan and reimbursements for travel and housing related to travel to Corporate headquarters. Each of our NEOs had 401(k) matching contributions in the amount of $12,200. Travel and housing reimbursements related to travel to Corporate headquarters were $4,752 for Mr. Watanabe, $8,117 for Mr. Matsuda, $10,290 for Mr. Burnett, $15,366 for Mr. Lock and $0 for Mr. Burrows.
(4)Mr. Matsuda commenced services with us in January 2022.
(5)Mr. Matsuda’s stock awards include 39,000 performance-based stock units that begin vesting upon the achievement of certain milestones. The milestone for 19,500 of such performance-based stock units was achieved on May 27, 2022, at which point 25% of the applicable grant vested and the remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period. The milestone for the remaining 19,500 of such performance-based stock units was achieved on November 18, 2022, at which point 25% of the applicable grant vested and the remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period.
(6)Mr. Burnett’s stock awards relate to 33,500 performance units that begin vesting upon achievement of certain FDA related milestones. One milestone was achieved on December 30, 2021 and, accordingly, 16,750 shares began vesting on that date, annually over a four-year period. The second milestone was achieved on August 18, 2022 and, accordingly, 16,750 shares began vesting on that date, annually over a four-year period.
(7)Mr. Burrows commenced services with us in May 2019 and was promoted to Chief Financial Officer on April 1, 2021.
(8)Mr. Burrows option awards include $800,000 related the achievement of certain financing-related milestones, which were completed on December 30, 2021, upon which date these awards began vesting monthly over a four-year period.
Grants of Plan-Based Awards Table
The following table provides information about the plan-based awards granted in 2022 to each of our NEOs.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards ($)(1) | | Estimated Future Payouts Under Equity Incentive Plan Awards ($)(2) | | All Other
Stock
Awards:
Number of
Shares of
Stock or
Units | | All Other
Option
Awards:
Number of
Securities
Underlying
Options | | Exercise or
Base Price
of
Awards | | Grant Date
Fair Value
of Stock
Options
and Awards ($) (3) |
| | Grant Date | | Target | Maximum | | Target | Maximum | | | | |
| NEO | | | Cash bonus | | Performance Awards | | RSU’s | | Options | | |
| Todd Franklin | | | | 354,000 | 531,000 | | | | | | | | | | | |
| Watanabe | | 3/1/2022 | | | | | | | | 70,900 | | | | | | | 1,252,803 | |
| | 3/1/2022 | | | | | | | | | | 188,100 | | | 17.67 | | | 2,314,700 | |
| Masaru | | | | 166,000 | 249,000 | | | | | | | | | | | |
| Matsuda, J.D. | | 1/3/2022 | (4) | | | | | | | | | 185,000 | | | 22.79 | | | 2,958,334 | |
| | 1/3/2022 | (4) | | | | 19,500 | | 19,500 | | | | | | | | | 444,405 | |
| | 1/3/2022 | (4) | | | | 19,500 | | 19,500 | | | | | | | | | 444,405 | |
| | 3/1/2022 | | | | | | | | 13,300 | | | | | | | 235,011 | |
| | 3/1/2022 | | | | | | | | | | 35,300 | | | 17.67 | | | 434,391 | |
| Patrick Burnett | | | | 182,867 | 274,300 | | | | | | | | | | | |
| M.D., Ph.D. | | 3/1/2022 | | | | | | | | 17,800 | | | | | | | 314,526 | |
| | 3/1/2022 | | | | | | | | | | 47,100 | | | 17.67 | | | 579,598 | |
| Ken Lock | | | | 157,000 | 235,500 | | | | | | | | | | | |
| | 3/1/2022 | | | | | | | | 13,300 | | | | | | | 235,011 | |
| | 3/1/2022 | | | | | | | | | | 35,300 | | | 17.67 | | | 434,391 | |
| Scott Burrows | | | | 154,667 | 232,000 | | | | | | | | | | | |
| | 3/1/2022 | | | | | | | | 13,300 | | | | | | | 235,011 | |
| | 3/1/2022 | | | | | | | | | | 35,300 | | | 17.67 | | | 434,391 | |
(1)Amounts represent cash-based annual incentive bonuses for fiscal year 2022, following the achievement of certain corporate goals as determined by the Compensation Committee and as detailed in the heading “Compensation Discussion & Analysis – Evaluation of Performance Against 2022 Corporate Goals”. The “maximum” amounts shown in the table above reflect the largest possible payments related to 2022, to be paid in March 2023. There are no minimum thresholds.
(2)Amounts represent performance-based stock awards. The number of units granted equals the target number and maximum number of units of the award. There are no minimum thresholds.
(3)Amounts represent the aggregate grant date fair values of the equity awards calculated in accordance with ASC Topic 718. The aggregate grant date fair value for the RSUs was based on the fair value of our common stock on the date of grant, which was determined as the closing market price per share of our common stock on the date of grant. The aggregate grant date fair value for the Stock Options and Performance Awards was based on the Black-Scholes option valuation methodology. This calculation is performed for accounting purposes and reported in the table. However, our NEOs may never realize any value from their equity awards. For additional information, refer to Note 10 of the audited financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 for the assumptions used in calculating these amounts. The amount reported above for Performance Awards includes the probable outcome of the performance measures being achieved, which was also the highest level of achievement of the performance conditions for Mr. Matsuda.
(4)Mr. Matsuda joined the Company in January 2022 and was awarded new-hire stock options and performance-based stock awards comprised of 39,000 performance-based stock units. The performance-based stock awards were subject to the achievement of certain milestones. The milestone for 19,500 of such performance-based stock units was achieved on May 27, 2022, at which point 25% of the applicable grant vested and the remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period. The milestone for the remaining 19,500 of such performance-based stock units was achieved on November 18, 2022, at which point 25% of the applicable grant vested and the remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period.The grant-date fair value of the performance-based stock awards were based on the maximum value to be attributed to the awards.
Outstanding Equity Awards at 20202022 Fiscal Year End.
The following table lists all outstanding equity awards held by our NEOs as of December 31, 2020.2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option Awards | | Stock Awards |
| Name | | Vesting Commencement Date | | Number of Securities Underlying Unexercised Options Exercisable (#) | | Number of Securities Underlying Unexercised Options Unexercisable (#) | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options(#) | | Option Exercise Price (#) | | Option Expiration Date | | Number of Shares or Units of Stock That Have Not Vested (#) | | Market Value of Shares or Units of Stock That Have Not Vested ($)(1) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(1) |
| Todd Franklin Watanabe | | (3) | | | | | | | | | | | | 35,833 (2) (3) | | 1,007,982 | | | | |
| | (4) | | | | | | | | | | | | 60,984 (2) (4) | | 1,715,480 | | | | |
| | (5) | | | | | | | | | | | | 44,605 (2) (5) | | 1,254,739 | | | | |
| | (6) | | 155,492 | | | | | | 1.68 | | 03/13/29 | | | | | | | | |
| | (7) | | 24,991 | | | | | | 6.52 | | 11/20/29 | | | | | | | | |
| | (8) | | 217,973 | | | | | | 1.68 | | 03/13/29 | | | | | | | | |
| | (9) | | 65,000 | | | | | | 27.61 | | 02/27/30 | | | | | | | | |
| | (10) | | | | | | | | | | | | 29,000 | | 815,770 | | | | |
| Patrick E. Burnett | | (11) | | | | 320,000 | | | | 25.89 | | 08/03/30 | | | | | | | | |
| | (12) | | | | | | | | | | | | | | | | 33,500 | | 942,355 |
| Kenneth A. Lock | | (13) | | 147,080 | | | | | | 6.52 | | 10/28/29 | | | | | | | | |
| | (9) | | 27,000 | | | | | | 27.61 | | 02/27/30 | | | | | | | | |
| | (10) | | | | | | | | | | | | 9,000 | | 253,170 | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option Awards | | Stock Awards |
| Name | | Vesting Commence-ment Date | | Number of Securities Underlying Unexercised Options Exercisable (#) | | Number of Securities Underlying Unexercised Options Unexercisable (#) | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options(#) | | Option Exercise Price (#) | | Option Expiration Date | | Number of Shares or Units of Stock That Have Not Vested (#) (1) | | Market Value of Shares or Units of Stock That Have Not Vested ($)(2) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(1) |
| Todd | | (3) | | | | | | | | | | | | 14,853 | | 219,824 | | | | |
| Franklin | | (4) | | 109,348 | | | | | | 1.68 | | 03/13/29 | | | | | | | | |
| Watanabe | | (5) | | 43,400 | | 55,800 | | | | 32.44 | | 03/02/31 | | | | | | | | |
| | (6) | | 65,000 | | | | | | 27.61 | | 02/26/30 | | | | | | | | |
| | (7) | | 24,991 | | | | | | 6.52 | | 11/20/29 | | | | | | | | |
| | (8) | | 217,973 | | | | | | 1.68 | | 03/13/29 | | | | | | | | |
| | (9) | | 35,270 | | 152,830 | | | | 17.67 | | 03/03/32 | | | | | | | | |
| | (10) | | | | | | | | | | | | 14,500 | | 214,600 | | | | |
| | (11) | | | | | | | | | | | | 24,525 | | 362,970 | | | | |
| | (12) | | | | | | | | | | | | 70,900 | | 1,049,320 | | | | |
| Masaru | | (13) | | | | 185,000 | | | | 22.79 | | 01/03/32 | | | | | | | | |
| Matsuda, | | (9) | | 6,619 | | 28,681 | | | | 17.67 | | 03/03/32 | | | | | | | | |
| J.D. | | (12) | | | | | | | | | | | | 13,300 | | 196,840 | | | | |
| | (14) | | | | | | | | | | | | 14,625 | | 216,450 | | | | |
| | (15) | | | | | | | | | | | | 14,625 | | 216,450 | | | | |
| Patrick | | (5) | | 12,600 | | 16,200 | | | | 32.44 | | 03/02/31 | | | | | | | | |
| Burnett, | | (16) | | 186,667 | | 133,333 | | | | 25.89 | | 08/02/30 | | | | | | | | |
| M.D., | | (9) | | 8,831 | | 38,269 | | | | 17.67 | | 03/03/32 | | | | | | | | |
| Ph.D. | | (11) | | | | | | | | | | | | 7,125 | | 105,450 | | | | |
| | (12) | | | | | | | | | | | | 17,800 | | 263,440 | | | | |
| | (17) | | | | | | | | | | | | 12,563 | | 185,932 | | | | |
| | (18) | | | | | | | | | | | | 16,750 | | 247,900 | | | | |
| Ken | | (5) | | 12,600 | | 16,200 | | | | 32.44 | | 03/02/31 | | | | | | | | |
| Lock | | (19) | | 147,080 | | | | | | 6.52 | | 10/28/29 | | | | | | | | |
| | (6) | | 27,000 | | | | | | 27.61 | | 02/26/30 | | | | | | | | |
| | (9) | | 6,619 | | 28,681 | | | | 17.67 | | 03/03/32 | | | | | | | | |
| | (10) | | | | | | | | | | | | 4,500 | | 66,600 | | | | |
| | (11) | | | | | | | | | | | | 7,125 | | 105,450 | | | | |
| | (12) | | | | | | | | | | | | 13,300 | | 196,840 | | | | |
| Scott L. | | (20) | | 7,705 | | 2,291 | | | | 6.52 | | 11/20/29 | | | | | | | | |
| Burrows | | (21) | | 29,988 | | 7,289 | | | | 1.68 | | 06/11/29 | | | | | | | | |
| | (22) | | 708 | | 292 | | | | 27.61 | | 02/26/30 | | | | | | | | |
| | (23) | | 9,583 | | 13,417 | | | | 29.74 | | 04/01/31 | | | | | | | | |
| | (5) | | 3,719 | | 4,781 | | | | 32.44 | | 03/02/31 | | | | | | | | |
| | (24) | | 13,926 | | 41,778 | | | | 20.59 | | 08/23/31 | | | | | | | | |
| | (9) | | 6,619 | | 28,681 | | | | 17.67 | | 03/03/32 | | | | | | | | |
| | (10) | | | | | | | | | | | | 2,000 | | 29,600 | | | | |
| | (11) | | | | | | | | | | | | 2,100 | | 31,080 | | | | |
| | (12) | | | | | | | | | | | | 13,300 | | 196,840 | | | | |
(1)Based on the closing price of our common stock on December 31, 2020 of $28.13 per share.
(2)Each award is subject to the acceleration of vesting provisions in each named executive officers’ severance & change in control agreement.
(3)The restricted stock was acquired through(2)Based on the early exercise of a stock option at an exerciseclosing price of $0.36our common stock on December 30, 2022 (the last trading day in 2022) of $14.80 per share. The restricted stock vests monthly over a four year period beginning November 8, 2016, subject to the holder’s continuous provision of services to us on each vesting date.
(4)The restricted stock was acquired through the early exercise of a stock option at an exercise price of $0.36 per share. The restricted stock vests monthly over a four year period beginning March 1, 2018, subject to the holder’s continuous provision of services to us on each vesting date.
(5)(3)The restricted stock was acquired through the early exercise of a stock option at an exercise price of $1.68 per share. The restricted stock was granted on March 31,13, 2019 and was subject to a performance objective, which was achieved on December 20, 2019. The restricted stock vests monthly over a four year period beginning December 20, 2019, subject to the holder’s continuous provision of services to us on each vesting date.
(6)(4)The option was granted on March 13, 2019 and was subject to a performance objective, which was achieved on December 20, 2019. The option vests monthly over a four year period beginning December 20, 2019, subject to the optionee’s continuous provision of services to us through each such date. The option contains an early-exercise provision and is exercisable as to unvested shares, subject to our right of repurchase. In addition to the foregoing vesting arrangements, the option is subject to acceleration upon certain events pursuant to the terms of the named executive officers’ severance & change in control agreement.
(5)The option vests monthly over a four year period beginning March 3, 2021, subject to the optionee’s continuous provision of services to us through each such date.
(6)The option vests monthly over a four year period beginning February 27, 2020, subject to the optionee’s continuous provision of services to us through each such date. The options contain an early-exercise provision and are exercisable as to unvested shares, subject to our right of repurchase.
(7)The option vests monthly over a four year period beginning November 20, 2019, subject to the optionee’s continuous provision of services to us through each such date. The option contains an early-exercise provision and is exercisable as to unvested shares, subject to our right of repurchase.
(8)The option was granted on March 13, 2019 and was subject to a performance objective, which was achieved on January 30, 2020. The option vestsoptions vest monthly over a four year period beginning January 30, 2020, subject to the optionee’s continuous provision of services to us through each such date. The option contains an early-exercise provision and is exercisable as to unvested shares, subject to our right of repurchase.
(9)The option vests monthly over a four year period beginning February 27, 2020,March 1, 2022, subject to the optionee’s continuous provision of services to us through each such date. The option contains an early-exercise provision and is exercisable as to unvested shares, subject to our right of repurchase.
(10)The restricted stock vests 1/4 annually over a period of four years beginning February 27, 2020.2020, subject to the holder’s continuous provision of services to us through each such date.
(11)The restricted stock vests 1/4 annually over a period of four years beginning March 3, 2021, subject to the holder’s continuous provision of services to us through each such date.
(12)The restricted stock vests 1/4 annually over a period of four years beginning March 1, 2022, subject to the holder’s continuous provision of services to us through each such date.
(13)The option vests 1/4 after one year and monthly over a four year period beginning January 3, 2022, subject to the optionee’s continuous provision of services to us through each such date.
(14)The performance stock award was granted on January 3, 2022 and was subject to a performance objective, which was achieved on May 27, 2022, at which point 25% of the applicable grant vested. The remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period, subject to the holder’s continuous provision of services to us through each such date.
(15)The performance stock award was granted on January 3, 2022 and was subject to a performance objective, which was achieved on November 18, 2022, at which point 25% of the applicable grant vested. The remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period, subject to the holder’s continuous provision of services to us through each such date.
(16)The option vests 1/4 after one year and monthly over a four year period beginning August 1, 2020, subject to the optionee’s continuous provision of services to us through each such date.
(12)(17)The performance stock award was granted on August 3, 2020 and will begin vesting ¼ annuallywas subject to an FDA related milestone performance objective, which was achieved on December 30, 2021. The shares vest over a four yearfour-year period beginning uponannually on each anniversary of the achievement date.
(18)The performance stock award was granted on August 3, 2020 and was subject to an FDA related milestone performance objective, which was achieved on August 18, 2022. The shares vest over a four-year period annually on each anniversary of a performance objective.the achievement date.
(13)(19)The option vests 1/4 after one year and monthly over a four year period beginning October 14, 2019, subject to the optionee’s continuous provision of services to us through each such date. The option contains an early-exercise provision and is exercisable as to unvested shares, subject to our right of repurchase.
(20)The option vests monthly over a four year period beginning November 20, 2019, subject to the optionee’s continuous provision of services to us through each such date.
(21)The option vests 1/4 after one year and monthly over a four year period beginning May 22, 2019, subject to the optionee’s continuous provision of services to us through each such date.
(22)The option vests monthly over a four year period beginning February 27, 2020, subject to the optionee’s continuous provision of services to us through each such date.
(23)The option vests monthly over a four year period beginning April 1, 2021, subject to the optionee’s continuous provision of services to us through each such date.
(24)The option was granted on August 24, 2021 and was subject to a financing related performance objective, which was achieved on December 30, 2021. The option vests monthly over a four year period beginning December 30, 2021, subject to the optionee’s continuous provision of services to us through each such date.
NarrativeOption Exercises and Stock Vested in Fiscal 2022
The following table summarizes the exercise of options and the vesting of RSUs for each of our NEOs during the year ended December 31, 2022.
| | | | | | | | | | | | | | | | | | | | |
| | Option Awards | | Stock Awards |
| Named Executive Officer | | Number of Securities Acquired on Exercise(1) | Value Realized on Exercise ($)(2) | | Number of Securities Acquired on Vesting (3) | Value Realized on Vesting ($)(4) |
| Todd Franklin Watanabe | | 12,287 | | 271,886 | | | 15,425 | | 274,880 | |
Masaru Matsuda, J.D. (5) | | — | | — | | | 9,750 | | 193,586 | |
| Patrick Burnett, M.D., Ph.D. | | — | | — | | | 6,562 | | 103,934 | |
| Kenneth Lock | | — | | — | | | 4,625 | | 82,444 | |
| Scott Burrows | | 5,000 | | 78,347 | | | 1,700 | | 30,359 | |
(1)These amounts represent the exercise of stock options granted in March 2019 and June 2019 for Messrs. Watanabe and Burrows, respectively. For Mr. Watanabe, 4,326 shares were withheld by the Company to 2020 Summary Compensation Tablecover the option exercise price and Outstanding Equity Awardstax withholding.
(2)The value shown is based on the stock options exercised multiplied by the difference between the price at 2020 Fiscal Year Endwhich they were valued on the date of exercise and the stock option exercise price.
Executive Compensation Philosophy & Compensation Mix(3)For Messrs. Watanabe, Matsuda, Burnett, Lock and Burrows, 4,712, 3,515, 2,486, 1,958 and 723 shares, respectively, were withheld by the Company to cover the tax withholdings.
We believe(4)The value shown is the closing price of a share of our executive compensation program is closely aligned with stockholders’ interests. While base salaryCommon Stock on the vesting date of RSUs, multiplied by the number of units vested/paid.
(5)Mr. Matsuda joined the Company in January 2022 and an annualwas awarded new-hire stock options and performance-based cash incentive opportunities incentivizestock awards comprised of 39,000 performance-based stock units. The performance-based stock awards were subject to the achievement of shorter-term goals, our long-term equitycertain milestones. The milestone for 19,500 of such performance-based stock units was achieved on May 27, 2022, at which point 25% of the applicable grant vested and the remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period. The milestone for the remaining 19,500 of such performance-based stock units was achieved on November 18, 2022, at which point 25% of the applicable grant vested and the remaining portion of the grant will vest in equal 25% installments on each subsequent one year anniversary over a remaining three-year period.The grant-date fair value of the performance-based stock awards represent a longer-term compensation structure that promotes retention and continuous commitmentwere based on the maximum value to be attributed to the operating resultsawards.
Pension Benefits
We do not have a defined benefit plan. Our named executive officers did not participate in, or otherwise receive, any special benefits under, any pension or defined benefit retirement plan sponsored by us during 2022.
Nonqualified Deferred Compensation
During 2022, our named executive officers did not contribute to, or earn any amount with respect to, any defined contribution or other plan sponsored by us that provides for the deferral of compensation on a basis that is not tax-qualified.
Equity Compensation Plan Information
The following table provides certain information as of December 31, 2022, with respect to all of our equity compensation plans in effect on that date.
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) (1) | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights (2) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a) (6) |
| Equity Compensation Plans Approved by Stockholders (3) (4) (5) | | 9,052,752 | | $19.93 | | 3,784,386 |
| Equity Compensation Plans Not Approved by Stockholders | | — | | — | | — |
| Total | | 9,052,752 | | $19.93 | | 3,784,386 |
(1)Amounts include 7,476,223 options outstanding and 1,576,529 RSU’s outstanding under the Arcutis Biotherapeutics, Inc. 2022 Employment Inducement Incentive Plan (the “2022 Plan”), 2020 Equity Incentive Plan (2020 Plan) and 2017 Equity Incentive Plan (2017 Plan).
(2)The weighted-average exercise price is calculated based solely on the exercise prices of the Company. We further believe this compensation mix rewardsoutstanding options and does not reflect shares that will be issued upon the vesting of outstanding RSUs, which have no price.
(3)Includes the 2017 Plan, the 2020 Plan, the 2020 Employee Stock Purchase Plan (the “2020 ESPP”) and the 2022 Plan.
(4)The 2020 Plan contains an “evergreen” provision, pursuant to which the number of shares of common stock reserved for issuance or transfer pursuant to awards under the 2020 Plan shall be increased on the first day of each executive, including the NEOs, for their individual contributionsyear beginning in 2021 and ending in 2030, equal to the Company, both present and future. At this phase in our growth cycle, a majoritylesser of (i) four percent (4.0%) of the annual total direct compensationshares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year, and (ii) such smaller number of shares of stock as determined by our NEOs is directly tied, throughBoard; provided, however, that no more than 11,000,000 shares of stock may be issued upon the useexercise of equity awards,incentive stock options.
(5)The 2020 ESPP contains an “evergreen” provision, pursuant to which the growth in the valuemaximum number of shares of our common stock.stock authorized for sale under the 2020 ESPP shall be increased on the first day of each year beginning in 2021 and ending in 2030, equal to the lesser of (i) one percent (1.0%) of the shares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year, and (ii) such number of shares of common stock as determined by our Board; provided, however, no more than 5,265,000 shares of our common stock may be issued thereunder.
Executive(6)Includes 1,069,711 shares that were available for future issuance under the 2020 ESPP.
DIRECTOR COMPENSATION
Non-Employee Director Compensation ProcessProgram
We maintain a compensation program that provides cash and equity compensation to our non-employee directors (the “Non-Employee Director Compensation Program”) for their service on the Board and its committees as discussed below. The Non-Employee Director Compensation Program was initially adopted and approved by our Board in connection with our initial public offering effective January 30, 2020, and is intended to be fair and competitive to account for the time and effort required of our directors. We do not provide directors who are also our employees any additional compensation for their service as directors.
The Compensation Committee overseesregularly reviews the Non-Employee Director Compensation Program with assistance from Pay Governance, which prepares a comprehensive assessment of our program. This assessment includes benchmarking of director compensation against the same peer group used for executive compensation program (including our executive compensation policiespurposes and practices), administers our various equity plans and approves or makes recommendations regarding the compensation of our executive officers, including our NEOs to the Board. The Compensation Committee reviews the performance of each NEO to determine whether to make any changes to theiran update on recent trends in director compensation. The Compensation Committee presents its recommendations to our Board forFollowing such a review, and final approval.
Our Chief Executive Officer makes recommendations to the Compensation Committee regardingapproved changes to the salary, annual cash incentive award, and equity awards forcompensation in the executive officers other than himself, including the NEOs. At theNon-Employee Director Compensation Committee’s request, our Chief Executive Officer reviews withProgram, effective June 2021. Following a review in 2022, the Compensation Committee approved changes to the individual performanceequity compensation whereby each non-employee director receives an annual grant consisting of a mix of stock options and restricted stock units, effective June 2022.
Cash Compensation
Pursuant to the Non-Employee Director Compensation Program, each non-employee director receives an annual retainer of $40,000 (the “Base Retainer”). Non-employee directors are eligible to receive additional annual retainers as follows:
| | | | | | | | | | | |
| | | Amount |
| Board Chair | | | $35,000 |
| Audit Committee Chair | | | 20,000 |
| Compensation Committee Chair | | | 15,000 |
| Nominating and Corporate Governance Committee Chair | | | 10,000 |
| Audit Committee Member | | | 10,000 |
| Compensation Committee Member | | | 7,500 |
| Nominating and Corporate Governance Committee Member | | | 5,000 |
The cash compensation set forth above is payable in equal quarterly installments, in arrears following the end of each quarter in which the service occurred, pro-rated for any partial months of the other executive officers, including each of our NEOs. The Compensation Committee gives considerable weight to our Chief Executive Officer’s evaluations and determines whether the recommended changes in each executive officer’s compensation, if any, are appropriate.
The Compensation Committee receives support from our Human Resources Department in designing our executive compensation program and analyzing competitive market practices. In addition, our Chief Executive Officer participates in Compensation Committee meetings, providing input from our executive team on organizational structure, executive development, and financial analysis.
While the Compensation Committee does not establish compensation levels based solely on a review of competitive market data, it believes that such data is a useful tool in its deliberations as it recognizes that our compensation policies and practices must be competitive in the marketplace for us to be able to attract, motivate, and retain qualified executive officers. Generally, the Compensation Committee reviews our executive compensation relative to our established competitive market (based on an analysis of the compensation policies and practices of a select group of peer companies) every year. The Compensation Committee uses the competitive market data when evaluating all aspects of executive compensation. The Compensation Committee engages Pay Governance LLC to assist with updating our compensation peer group and assessing the competitiveness of our executive compensation program.
2020 Salariesservice.
We use base salaryhave also reimbursed, and will continue to compensatereimburse, our NEOsnon-employee directors for their experience, skills, knowledge, roletravel, lodging, and responsibilities. When establishing the base salariesother reasonable expenses incurred in attending meetings of our NEOs, our Board and the Compensation Committee consider a variety of factors, including each NEO’s seniority and level of responsibility as well as competitive market data and our ability to find a replacement if the individual left our employment. The base salary of each NEO is reviewed annually and adjusted from time to time to reflect performance and realign with market data. In March 2020, upon recommendation of the Compensation Committee, the Board approved increasing Mr. Watanabe’s annual base salary from $400,000 to $450,000 and Mr. Lock’s annual base salary from $310,000 to $340,000. Dr. Burnett’s annual base salary of $410,000 was approved by the Board when he joined the company in August 2020, pro-rated for his partial service in 2020.
2020 Incentive Compensation
We use cash incentive compensation to motivate our NEOs to achieve our annual operational objectives, while making progress towards our longer-term growth and other corporate goals. At the beginning of each year, typically in January, the Board approves a set of technical, operational and financial goals for the Company for that year which are key drivers in determining the eventual cash incentive compensation for that year. The Compensation Committee recommends annual cash incentive compensation targets for our NEOs to our Board for its consideration and approval. Each NEO’s target cash incentive is expressed as a percentage of base salary which can be achieved by meeting corporate goals at target level. The 2020 annual cash incentive targets for Mr. Watanabe, Mr. Lock and Dr. Burnett were set at 50%, 40% and 40% of their respective base salaries.
For 2020, our NEOs were eligible to earn annual cash incentives based on the achievement of certain corporate performance objectives approved by the Compensation Committee and the Board. For 2020, the Board set corporate performance goals in the three broad strategic areas of (i) advancing the pipeline, (ii) ensuring continuous clinical supply, and (iii) financing the business. Each area included specific performance objectives and a corresponding weighting. For each strategic area, the Board also approved certain “stretch” goals with corresponding weightings, such that the corporate goals could be achieved at up to 150% of target. The entirety of Mr. Watanabe’s, Mr. Lock’s and Dr. Burnett’s annual cash incentives was determined by the Company’s performance. In January 2021, the Board reviewed and approved the achievementcommittees of our 2020 corporate goals at 127.5%. Based on this level of achievement, our NEOs were paid at the following percentages of their base salary earned in 2020 (except in the case of Dr. Burnett whose annual bonus was paid based on his annual base salary for 2020 and not on base salary actually earned in 2020): Mr. Watanabe: 63.75%; Mr. Lock: 51%; and Dr. Burnett: 51%.Board.
The actual annual cash incentives awarded to each NEO for 2020 performance are set forth above in the Summary Compensation Table in the column titled “Non-Equity Incentive Plan Compensation.”
Equity Compensation Plan Information
The following table provides certain information as of December 31, 2022, with respect to all of our equity compensation plans in effect on that date.
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) (1) | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights (2) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a) (6) |
| Equity Compensation Plans Approved by Stockholders (3) (4) (5) | | 9,052,752 | | $19.93 | | 3,784,386 |
| Equity Compensation Plans Not Approved by Stockholders | | — | | — | | — |
| Total | | 9,052,752 | | $19.93 | | 3,784,386 |
(1)Amounts include 7,476,223 options outstanding and 1,576,529 RSU’s outstanding under the Arcutis Biotherapeutics, Inc. 2022 Employment Inducement Incentive Plan (the “2022 Plan”), 2020 Equity Incentive Plan (2020 Plan) and 2017 Equity Incentive Plan (2017 Plan).
(2)The weighted-average exercise price is calculated based solely on the exercise prices of the outstanding options and does not reflect shares that will be issued upon the vesting of outstanding RSUs, which have no price.
(3)Includes the 2017 Plan, the 2020 Plan, the 2020 Employee Stock Purchase Plan (the “2020 ESPP”) and the 2022 Plan.
(4)The 2020 Plan contains an “evergreen” provision, pursuant to which the number of shares of common stock reserved for issuance or transfer pursuant to awards under the 2020 Plan shall be increased on the first day of each year beginning in 2021 and ending in 2030, equal to the lesser of (i) four percent (4.0%) of the shares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year, and (ii) such smaller number of shares of stock as determined by our Board; provided, however, that no more than 11,000,000 shares of stock may be issued upon the exercise of incentive stock options.
(5)The 2020 ESPP contains an “evergreen” provision, pursuant to which the maximum number of shares of our common stock authorized for sale under the 2020 ESPP shall be increased on the first day of each year beginning in 2021 and ending in 2030, equal to the lesser of (i) one percent (1.0%) of the shares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year, and (ii) such number of shares of common stock as determined by our Board; provided, however, no more than 5,265,000 shares of our common stock may be issued thereunder.
(6)Includes 1,069,711 shares that were available for future issuance under the 2020 ESPP.
DIRECTOR COMPENSATION
Non-Employee Director Compensation Program
We usemaintain a compensation program that provides cash and equity awardscompensation to motivate and reward our executive officersnon-employee directors (the “Non-Employee Director Compensation Program”) for long-term corporate performance basedtheir service on the value ofBoard and its committees as discussed below. The Non-Employee Director Compensation Program was initially adopted and approved by our Board in connection with our initial public offering effective January 30, 2020, and is intended to be fair and competitive to account for the Company’s common stocktime and thereby, align the interestseffort required of our executive officersdirectors. We do not provide directors who are also our employees any additional compensation for their service as directors.
The Compensation Committee regularly reviews the Non-Employee Director Compensation Program with thoseassistance from Pay Governance, which prepares a comprehensive assessment of our shareholders. We believeprogram. This assessment includes benchmarking of director compensation against the same peer group used for executive compensation purposes and an update on recent trends in director compensation. Following such a review, the Compensation Committee approved changes to the cash and equity provides appropriate long-term incentive and retentioncompensation in the Non-Employee Director Compensation Program, effective June 2021. Following a review in 2022, the Compensation Committee approved changes to the equity compensation whereby each non-employee director receives an annual grant consisting of our executive officers.
In February, 2020, we made the following grantsa mix of stock options and restricted stock units, to our named executive officers:effective June 2022.
| | | | | | | | | | | | | | |
| NEO | | Number of Shares Underlying Options | | Restricted Stock Units |
| Franklin Todd Watanabe | | 65,000 | | 29,000 |
| Kenneth A. Lock | | 27,000 | | 9,000 |
One-forty-eight (1/48th) of the shares shall vest on each monthly anniversary of the vesting commencement date, subject to continued service on each applicable vesting date. One fourth (1/4th) of the restricted stock units shall vest annually beginning on the one year anniversary of the vesting commencement date, subject to continued service on each applicable vesting date.
In August, 2020, Dr. Burnett received the following grants upon commencement employment as our Chief Medical Officer:
| | | | | | | | | | | | | | |
| NEO | | Number of Shares Underlying Options | | Performance Stock Units |
| Patrick E. Burnett, M.D., Ph.D. | | 320,000 | | 33,500 |
One fourth (1/4th) of the shares subjectPursuant to the options shall vest on the twelve (12) month anniversaryNon-Employee Director Compensation Program, each non-employee director receives an annual retainer of the vesting commencement date, and one-forty-eight(1/48th) of the shares shall vest on each monthly anniversary of the vesting commencement date thereafter, subject to continued service on each applicable vesting date. The performance stock units will commence vesting based on the acceptance or approval by the U.S. Food and Drug Administration of certain applications. One-fourth (1/4th$40,000 (the “Base Retainer”) of the performance stock units will begin vesting on the one-year anniversary of the achievement of the applicable performance milestones and on each yearly anniversary thereafter, subject to continued service on each applicable vesting date.
Other Elements of Compensation
Retirement Plan
We maintain a 401(k) retirement savings plan for our employees, including our NEOs, who satisfy certain eligibility requirements. Our NEOs. Non-employee directors are eligible to participatereceive additional annual retainers as follows:
| | | | | | | | | | | |
| | | Amount |
| Board Chair | | | $35,000 |
| Audit Committee Chair | | | 20,000 |
| Compensation Committee Chair | | | 15,000 |
| Nominating and Corporate Governance Committee Chair | | | 10,000 |
| Audit Committee Member | | | 10,000 |
| Compensation Committee Member | | | 7,500 |
| Nominating and Corporate Governance Committee Member | | | 5,000 |
The cash compensation set forth above is payable in equal quarterly installments, in arrears following the 401(k) plan on the same terms as other full-time employees. We began making matching safe-harbor contributions under our 401(k) plan effective April 1, 2020 at 100% of the first 3%end of each participant’s contributions, plus 50%quarter in which the service occurred, pro-rated for any partial months of each participant’s next 2% of contributions,service.
We have also reimbursed, and will continue to reimburse, our non-employee directors for a maximum aggregatematching contribution of 4%. We believe that providing a vehicle for tax-deferred retirement savings though our 401(k) plan adds to the overall desirabilitytheir travel, lodging, and other reasonable expenses incurred in attending meetings of our executive compensation packageBoard and further incentivizes our employees, including our NEOs, in accordance with our compensation policies.
Perquisites, Reimbursements and Other Benefits
Allcommittees of our full-time employees, including our NEOs, are eligible to participate in our health and welfare plans, including medical, dental and vision benefits, medical and dependent care flexible spending accounts, short-term and long-term disability insurance and life insurance. We do not provide our NEOs with perquisites or other personal benefits, other than the retirement, health and welfare benefits that apply uniformly to all of our employees.Board.
Executive Compensation Arrangements
During 2020, we were party to employment agreements with each of our NEOs, which provide for base salaries, target cash incentives, benefit plan participation, as well as certain additional benefits, as described below.
Todd Franklin Watanabe
We entered into a continued employment agreement with Mr. Watanabe, our President and Chief Executive Officer, in January 2020 (the “Watanabe Continued Employment Agreement”). The Watanabe Continued Employment Agreement provides for an annual base salary of $400,000, a target bonus of 35% of his actual base salary earned in a calendar year, and the opportunity to continue participating in the Company’s employee benefit plans. In a November 2020 First Amendment to Continued Employment Letter, we amended the agreement to indicate that Mr. Watanabe’s Principal Place of Employment is now in Utah and not California.
Under Mr. Watanabe’s severance and change in control agreement (the “Watanabe Severance and Change in Control Agreement”) that went into effect on the date of the Company’s initial public offering, he is entitled to certain benefits in the event of a change in control, termination of employment with cause, or resignation for good reason.
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Mr. Watanabe will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the Qualifying Termination for twelve (12) months following termination;
•Subject to Mr. Watanabe’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Mr. Watanabe with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Mr. Watanabe will be entitled to receive the following benefits:
•Severance pay in an amount equal to eighteen (18) months of his base salary rate in effect immediately prior to the Qualifying Termination plus 1.5 times his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over an eighteen-month period.
•Subject to Mr. Watanabe’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) eighteen (18) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Mr. Watanabe with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions, as well as the performance-based stock options granted on March 13, 2019.
•Payment for any earned but unpaid base salary and other vested cash entitlements, such as bonus earned and payable from a prior year.
Kenneth A. Lock
We entered into a continued employment agreement with Mr. Lock, our Senior Vice President and Chief Commercial Officer, in January 2020 (the “Lock Continued Employment Agreement”). The Lock Continued Employment Agreement provides for an initial annual base salary of $310,000, an initial target
bonus of 30% of his actual base salary earned in a calendar year, and the opportunity to participate in the Company’s employee benefit plans. In addition, the Lock Continued Employment Agreement also provides for reimbursement by the Company for travel expenses incurred for travel to and from, and housing, at the Company’s corporate headquarters, up to a maximum of $4,000 per month.
Under Mr. Lock’s severance and change in control agreement (the “Lock Severance and Change in Control Agreement”) that went into effect on the date of the Company’s initial public offering, he is entitled to certain benefits in the event of a change in control, termination of employment with cause, or resignation for good reason.
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Mr. Lock will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the Qualifying Termination for nine (9) months following termination;
•Subject to Mr. Lock’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) nine (9) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Mr. Lock with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Mr. Lock will be entitled to receive the following benefits:
•Severance pay in an amount equal to twelve (12) months of his base salary rate in effect immediately prior to the Qualifying Termination plus his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over a twelve-month period.
•Subject to Mr. Lock’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Mr. Lock with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions.
•Payment for any earned but unpaid base salary and other vested cash entitlements, such as bonus earned and payable from a prior year.
Patrick E. Burnett, M.D., Ph.D.
We entered into an executive employment agreement with Dr. Burnett, our Senior Vice President and Chief Medical Officer, in July 2020 (the “Burnett Employment Agreement”) in connection with him joining the Company in August 2020. The Burnett Employment Agreement provides for an initial annual base salary of $410,000, an initial target bonus of 40% of his actual base salary earned in a calendar year, and the opportunity to participate in the Company’s employee benefit plans. In addition, the Burnett Employment Agreement provides for reimbursement by the Company for travel expenses incurred for travel to and from, and housing, at the Company’s corporate headquarters, up to a maximum of $5,000 per month.
Under Dr. Burnett’s severance and change in control agreement (the “Burnett Severance and Change in Control Agreement”) that went into effect in July 2020, he is entitled to certain benefits in the event of a change in control, termination of employment with cause, or resignation for good reason.
In the event of a qualifying termination outside of a change in control period, including resignation for good reason, Dr. Burnett will be entitled to receive the following benefits:
•Severance pay in the form of continuation of his base salary rate in effect immediately prior to the Qualifying Termination for nine (9) months following termination;
•Subject to Dr. Burnett’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) nine (9) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Dr. Burnett with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
In the event of a qualifying termination (i) within eighteen (18) months following a change in control or (ii) within three (3) months preceding a change in control, Dr. Burnett will be entitled to receive the following benefits:
•Severance pay in an amount equal to twelve (12) months of his base salary rate in effect immediately prior to the Qualifying Termination plus his annual bonus amount for the then-current fiscal year based on 100% of target performance, paid out in substantially equal installments over a twelve-month period.
•Subject to Dr. Burnett’s timely election for continued coverage under COBRA, the Company shall pay, or reimburse, his monthly premium for him and his covered dependents under COBRA until the earliest of (a) twelve (12) months, (b) the date when he receives similar coverage with a new employer, or (c) the expiration of his continuation coverage under COBRA. The Company may elect that, in lieu of paying or reimbursing the premiums, the Company shall instead provide Dr. Burnett with a monthly cash payment equal to the amount the Company would have otherwise paid for his monthly premium, less applicable tax withholdings.
•Full acceleration and immediate exercisability, if applicable, of all unvested equity awards subject to time-based vesting conditions.
•Payment for any earned but unpaid base salary and other vested cash entitlements, such as bonus earned and payable from a prior year.
With respect to the NEO’s severance and change in control agreements:
“Cause” means the occurrence of any of the following events, as determined by the Company and/or our Board in its and/or their sole and absolute discretion: (i) executive engaging in any act of fraud, embezzlement or material act of dishonesty or misrepresentation with respect to the Company; (ii) executive’s violation of any federal or state law or regulation applicable to the business of the Company or its affiliates; (iii) executive’s material breach of any confidentiality agreement or assignment agreement between executive and the Company (or any affiliate of the Company); (iv) executive’s conviction of or plea of nolo contendere to a felony involving moral turpitude; (v) executive’s unauthorized use or disclosure of confidential information or trade secrets of the Company (or any parent, subsidiary or affiliate); (vi) any intentional misconduct by executive adversely affecting the business or affairs of the Company (or any parent, subsidiary or affiliate) in any material manner; (vii) executive has committed any breach of fiduciary or statutory duty that results in (or would reasonably be expected to result in) material harm to the Company; (viii) executive has breached any material term or condition of executive’s severance and change in control agreement or any other material agreement with or material policy of the Company; (ix) executive’s willful and repeated failure to perform in any material respect executive’s duties
hereunder after fifteen (15) days’ notice and an opportunity to cure such failure and a reasonable opportunity to present to our Board executive’s position regarding any dispute relating to the existence of such failure (other than on account of disability); or (x) executive’s failure to attempt in good faith to implement a clear and reasonable directive from the Company’s Chief Executive Officer (or our Board).
“Change in Control” means the occurrence of any of the following events: (i) any “person” (as such term is used in Sections 13(d) and 14(d) of the Exchange Act) becomes the “beneficial owner” (as defined in Rule 13d-3 of the Exchange Act), directly or indirectly, of securities of the Company representing more than fifty percent (50%) of the total voting power represented by the Company’s then outstanding voting securities; (ii) the consummation of the sale or disposition by the Company of all or substantially all of the Company’s assets; or (iii) the consummation of a merger or consolidation of the Company with any other corporation, other than a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or its parent) at least fifty percent (50%) of the total voting power represented by the voting securities of the Company or such surviving entity or its parent outstanding immediately after such merger or consolidation.
“Good Reason” means the occurrence of any of the following events or conditions, without executive’s express written consent: (i) a material diminution of executive’s base salary or target annual performance bonus; (ii) a material diminution in executive’s authority, duties or responsibilities; or (iii) any requirement by the Company that executive’s principal place of employment be relocated to a location more than fifty (50) miles from executive’s principal place of employment prior to such change, which relocation materially increases executive’s commuting distance. A termination of employment for Good Reason shall be effectuated by giving the Company written notice (“Notice of Termination for Good Reason”), setting forth in reasonable detail, the specific conduct of the Company that constitutes Good Reason and the specific provision(s) on which executive is relying. Notice of Termination for Good Reason must be provided within ninety (90) days of the condition first arising. The Company will have an opportunity to cure such conduct constituting Good Reason within thirty (30) days of receiving such Notice of Termination for Good Reason. If the Company does not cure such conduct within such thirty (30) day period, a termination of employment for Good Reason shall be effective on the thirty-first (31st) day following the date when the Notice of Termination for Good Reason is received by the Company.
Compensation Risk Assessment
Consistent with the SEC’s disclosure requirements, we have assessed our compensation programs for all employees. We have concluded that our compensation policies and practices do not create risks that are reasonably likely to have a material adverse effect on us.
The Compensation Committee monitors our compensation programs on an annual basis and expects to make modifications as necessary to address any changes in our business or risk profile.
Equity Compensation Plan Information
The following table provides certain information as of December 31, 2020,2022, with respect to all of our equity compensation plans in effect on that date.
| | Plan Category | Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) (1) | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights (2) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a) | Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) (1) | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights (2) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a) (6) |
| Equity Compensation Plans Approved by Stockholders (3) (4) (5) | Equity Compensation Plans Approved by Stockholders (3) (4) (5) | | 3,818,875 | | $12.09 | | 2,501,329 (6) | Equity Compensation Plans Approved by Stockholders (3) (4) (5) | | 9,052,752 | | $19.93 | | 3,784,386 |
| Equity Compensation Plans Not Approved by Stockholders | Equity Compensation Plans Not Approved by Stockholders | | — | | — | | — | Equity Compensation Plans Not Approved by Stockholders | | — | | — | | — |
| Total | Total | | 3,818,875 | | 12.09 | | 2,501,329 | Total | | 9,052,752 | | $19.93 | | 3,784,386 |
(1)Amounts include 3,655,9457,476,223 options outstanding and 162,9301,576,529 RSU’s outstanding under the Arcutis Biotherapeutics, Inc. 2022 Employment Inducement Incentive Plan (the “2022 Plan”), 2020 Equity Incentive Plan (2020 Plan) and 2017 Equity Incentive Plan (2017 Plan).
(2)The weighted-average exercise price is calculated based solely on the exercise prices of the outstanding options and does not reflect shares that will be issued upon the vesting of outstanding RSUs, which have no price.
(3)Includes the 2017 Plan, the 2020 Plan, and the 2020 Employee Stock Purchase Plan (the “2020 ESPP”). and the 2022 Plan.
(4)The 2020 Plan contains an “evergreen” provision, pursuant to which the number of shares of common stock reserved for issuance or transfer pursuant to awards under the 2020 Plan shall be increased on the first day of each year beginning in 2021 and ending in 2030, equal to the lesser of (i) four percent (4.0%) of the shares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year, and (ii) such smaller number of shares of stock as determined by our Board; provided, however, that no more than 11,000,000 shares of stock may be issued upon the exercise of incentive stock options.
(5)The 2020 ESPP contains an “evergreen” provision, pursuant to which the maximum number of shares of our common stock authorized for sale under the 2020 ESPP shall be increased on the first day of each year beginning in 2021 and ending in 2030, equal to the lesser of (i) one percent (1.0%) of the shares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year, and (ii) such number of shares of common stock as determined by our Board; provided, however, no more than 5,265,000 shares of our common stock may be issued thereunder.
(6)Includes 316,8121,069,711 shares that were available for future issuance under the 2020 ESPP.
DIRECTOR COMPENSATION
Non-Employee Director Compensation Program
We maintain a compensation program that provides cash and equity compensation to our non-employee directors (the “Non-Employee Director Compensation Program”) for their service on the Board and its committees as discussed below. The Non-Employee Director Compensation Program was initially adopted and approved by our Board in connection with our initial public offering effective January 30, 2020, and is intended to be fair and competitive to account for the time and effort required of our directors. We do not provide directors who are also our employees any additional compensation for their service as directors.
The Compensation Committee regularly reviews the Non-Employee Director Compensation Program with assistance from Pay Governance, which prepares a comprehensive assessment of our program. This assessment includes benchmarking of director compensation against the same peer group used for executive compensation purposes and an update on recent trends in director compensation. Following such a review, the Compensation Committee approved changes to the cash and equity compensation in the Non-Employee Director Compensation Program, effective June 2021. Following a review in 2022, the Compensation Committee approved changes to the equity compensation whereby each non-employee director receives an annual grant consisting of a mix of stock options and restricted stock units, effective June 2022.
Cash Compensation
Pursuant to the Non-Employee Director Compensation Program, each non-employee director receives an annual retainer of $40,000 (the “Base Retainer”). Non-employee directors are eligible to receive additional annual retainers as follows:
| | | | | | | | | | | |
| | | Amount |
| Board Chair | | | $35,000 |
| Audit Committee Chair | | | 20,000 |
| Compensation Committee Chair | | | 15,000 |
| Nominating and Corporate Governance Committee Chair | | | 10,000 |
| Audit Committee Member | | | 10,000 |
| Compensation Committee Member | | | 7,500 |
| Nominating and Corporate Governance Committee Member | | | 5,000 |
The cash compensation set forth above is payable in equal quarterly installments, in arrears following the end of each quarter in which the service occurred, pro-rated for any partial months of service.
We have also reimbursed, and will continue to reimburse, our non-employee directors for their travel, lodging, and other reasonable expenses incurred in attending meetings of our Board and committees of our Board.
Equity Compensation
The Non-Employee Director Compensation Program also provides for equity compensation to each non-employee director as compensation for his or her service on the Board. Upon a director’s initial appointment or election to our Board, such director automatically receives an award of stock options to purchase shares of our common stock having a grant date fair value of $500,000 (the “Initial Director Grant”). On an annual basis thereafter, each non-employee director is eligible to receive an award with a value equivalent to $250,000 consisting of (1) stock options having a grant date value of $162,500 (65% of $250,000) and (2) an award of restricted stock units having a grant date value of $87,500 (35% of $250,000) (the “Annual Director Grant”).
Each award granted under the Non-Employee Director Compensation Program has an exercise price per share equal to the closing trading price of our common stock on the date of grant. Initial Director Grants vest on each of the first three annual anniversaries of the grant date, subject to the award holder’s continued service through each applicable vesting date. Annual Director Grants vest in full on the earlier of the first anniversary of the grant date or immediately before the next annual meeting of stockholders, subject to the award holder’s continued service through the applicable vesting date. All equity awards granted to our non-employee directors under the Non-Employee Director Compensation Program will, to the extent they are outstanding and unvested,vest in full immediately prior to the consummation of a change in control.
Director Compensation Table
The following table sets forth information concerning the compensation earned by our non-employee directors during the year ended December 31, 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Fees Earned or Paid in Cash ($) | | Option Awards ($) (1) | | Stock Awards ($) (2) | | All Other Compensation ($) | | Total ($) |
| Bhaskar Chaudhuri, Ph.D. | | 59,667 | | 162,798 | | 87,490 | | — | | 309,955 |
| Terrie Curran | | 50,000 | | 162,798 | | 87,490 | | — | | 300,288 |
| Halley Gilbert | | 50,000 | | 162,798 | | 87,490 | | — | | 300,288 |
| Patrick J. Heron | | 82,500 | (3) | 162,798 | | 87,490 | | — | | 332,788 |
| Neha Krishnamohan | | 16,346 | | 499,502 | | 24,997 | | — | | 540,845 |
| Keith R. Leonard | | 47,500 | | 162,798 | | 87,490 | | — | | 297,788 |
| Sue-Jean Lin | | 53,621 | | 162,798 | | 87,490 | | — | | 303,909 |
| Joseph L. Turner | | 56,712 | | 162,798 | | 87,490 | | — | | 307,000 |
| Howard G. Welgus, M.D. | | 45,000 | | 162,798 | | 87,490 | | 8,625 | (4) | 303,913 |
(1)Amounts reflect the full grant date fair value of stock options granted during 2022 computed in accordance with ASC Topic 718, rather than the amounts paid to or realized by the named individual. See Note 10 of the audited financial statements included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 for the assumptions used in calculating these amounts.
(2)Amount reflects the full grant date fair value of restricted stock units granted during 2022.
(3)Amount paid to Frazier Healthcare Partners on behalf of Mr. Heron’s service as members of the Board.
(4)Amount represents consulting fees paid for services other than as a member of our Board.
As of December 31, 2022, outstanding options and restricted stock units held by our current non-employee directors were as follows:
| | | | | | | | | | | | | | |
| | Shares Subject to Outstanding Options | | Restricted Stock Units |
| Bhaskar Chaudhuri, Ph.D. | | 149,152 | | 4,312 |
| Terrie Curran | | 62,687 | | 4,312 |
| Halley Gilbert | | 62,687 | | 4,312 |
| Patrick J. Heron | | 49,187 | | 4,312 |
| Neha Krishnamohan | | 29,482 | | — |
| Keith R. Leonard | | 44,703 | | 4,312 |
| Sue-Jean Lin | | 37,519 | | 4,312 |
| Joseph L. Turner | | 62,687 | | 4,312 |
| Howard G. Welgus, M.D. (1) | | 137,201 | | 8,812 |
(1)Dr. Welgus received restricted stock units and stock options that were granted for his service as Chief Medical Officer, which continue to vest subject to his continued service as a member of our Board. 112,014 of his outstanding options and 4,500 of his outstanding RSU’s relate to his awards granted as CMO.
As of February 23, 2023, the Non-Employee Director Compensation Program has been further amended and restated, to permit the Board or the Compensation Committee, in its discretion, to provide each non-employee director with the opportunity to defer the issuance of the shares underlying RSUs granted under this program effective February 23, 2023.
INFORMATION ABOUT STOCK OWNERSHIP
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Security Ownership of Certain Beneficial Owners and Management
The following table presents information as to the beneficial ownership of our common stock as of April 13, 20213, 2023 for:
•each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock;
•each named executive officer as set forth in the summary compensation table above;
•each of our directors; and
•all executive officers and directors as a group.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Unless otherwise indicated below, to our knowledge, the persons and entities named in the table have sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable. Shares of our common stock subject to options that are currently exercisable or exercisable within 60 days of April 13, 20214, 2022 are deemed to be outstanding and to be beneficially owned by the person holding the stock options for the purpose of computing the percentage ownership of that person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
Percentage ownership of our common stock in the table is based on shares of our 50,149,74461,360,936 common stock issued and outstanding on April 13, 2021.3, 2023. This table is based upon information supplied by officers, directors and principal stockholders and Schedules 13D and Schedules 13G, if any, filed with the SEC. Unless otherwise indicated, the address of each of the individuals and entities named below is c/o Arcutis Biotherapeutics, Inc., 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Shares of Common Stock Beneficially Owned |
| Name of Beneficial Owner | | Common Stock | | Number of Shares Exercisable Within 60 Days | | Number of Shares Beneficially Owned | | Percentage of Shares Beneficially Owned |
| 5% and Greater Stockholders: | | | | | | | | |
| Bain Capital Life Sciences Entities (1) | | 3,979,292 | | — | | 3,979,292 | | 7.9% |
| Blackrock, Inc. (2) | | 2,895,054 | | — | | 2,895,054 | | 5.8% |
| FMR LLC (3) | | 6,545,164 | | — | | 6,545,164 | | 13.1% |
| Frazier Life Sciences VIII, L.P. (4) | | 10,542,790 | | — | | 10,542,790 | | 21.0% |
| Entities affiliated with OrbiMed (5) | | 6,073,850 | | — | | 6,073,850 | | 12.1% |
| Named Executive Officers and Directors: | | | | | | | | |
| Todd Franklin Watanabe (6) | | 751,603 | | 449,656 | | 1,201,259 | | 2.4% |
| Kenneth A. Lock (7) | | 15,417 | | 175,880 | | 191,297 | | * |
| Patrick E. Burnett, M.D., Ph.D. (8) | | — | | 1,800 | | 1,800 | | * |
| Howard G. Welgus, M.D. (9) | | 226,527 | | 120,744 | | 347,271 | | * |
| Bhaskar Chaudhuri, Ph.D. (10) | | 921,372 | | 57,321 | | 978,693 | | 1.9% |
| Terrie Curran | | — | | — | | — | | * |
| Halley Gilbert (11) | | — | | 37,500 | | 37,500 | | * |
| Joseph L. Turner (12) | | — | | 37,500 | | 37,500 | | * |
| Patrick J. Heron (13) | | 10,542,790 | | 24,000 | | 10,566,790 | | 21.1% |
| Jonathan T. Silverstein, J.D. (14) | | 6,073,850 | | 24,000 | | 6,097,850 | | 12.2% |
| Ricky Sun, Ph.D. (15) | | — | | 24,000 | | 24,000 | | * |
| All executive officers and directors as a group (17 persons) (16) | | 18,794,022 | | 1,232,759 | | 20,026,781 | | 39.0% |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Shares of Common Stock Beneficially Owned |
| Name of Beneficial Owner | | Common Stock | | Number of Shares Exercisable Within 60 Days | | Number of Shares Beneficially Owned | | Percentage of Shares Beneficially Owned |
| 5% and Greater Stockholders: | | | | | | | | |
FMR LLC(1) | | 9,139,173 | | | — | | | 9,139,173 | | | 14.9% |
Frazier Life Sciences VIII, L.P.(2) | | 8,684,232 | | | — | | | 8,684,232 | | | 14.2% |
Blackrock, Inc.(3) | | 5,214,258 | | | — | | | 5,214,258 | | | 8.5% |
State Street Global Advisors(4) | | 3,679,385 | | | — | | | 3,679,385 | | | 6.0% |
| Named Executive Officers and Directors: | | | | | | | | |
Todd Franklin Watanabe(5) | | 420,396 | | | 540,421 | | | 960,817 | | | 1.6% |
Masaru Matsuda, J.D. (6) | | 8,126 | | | 81,061 | | | 89,187 | | | * |
Patrick E. Burnett, M.D., Ph.D.(7) | | 8,299 | | | 260,474 | | | 268,773 | | | * |
Kenneth A. Lock (8) | | 25,454 | | | 203,465 | | | 228,919 | | | * |
Scott Burrows(9) | | 25,740 | | | 98,145 | | | 123,885 | | | * |
Howard G. Welgus, M.D.(10) | | 184,254 | | | 124,513 | | | 308,767 | | | * |
Bhaskar Chaudhuri, Ph.D.(11) | | 891,372 | | | 153,464 | | | 1,044,836 | | | 1.7% |
Terrie Curran(12) | | — | | | 54,499 | | | 54,499 | | | * |
Halley Gilbert(13) | | — | | | 66,999 | | | 66,999 | | | * |
Joseph L. Turner(14) | | — | | | 66,999 | | | 66,999 | | | * |
Patrick J. Heron(2)(15) | | 8,684,232 | | | 53,499 | | | 8,737,731 | | | 14.2% |
Keith Leonard(16) | | 1,750 | | | 27,077 | | | 28,827 | | | * |
Sue-Jean Lin(17) | | 300 | | | 24,682 | | | 24,982 | | | * |
Neha Krishnamohan(18) | | 1,232 | | | — | | | 1,232 | | | * |
All executive officers and directors as a group (17 persons)(19) | | 10,518,426 | | | 2,177,958 | | | 12,696,384 | | | 20.7% |
______________
*Indicates beneficial ownership of less than 1% of the total outstanding common stock.
(1)As reported on a Schedule 13D/A filed with the SEC on February 8, 2021. Consists of (i) 3,609,796 shares of our common stock held by Bain Capital Life Sciences Fund, L.P., or BC LS, and (ii) 369,496 shares of our common stock held by BCIP Life Sciences Associates, LP, or BCIP LS, and together with BC LS, the Bain Capital Life Sciences Entities. Bain Capital Life Sciences Investors, LLC, whose managers are Jeffrey Schwartz and Adam Koppel, is the ultimate general partner of BC LS and governs the investment strategy and decision-making process with respect to investments held by BCIP LS. AS a result, each of Bain Capital Life Sciences Investors, LLC, Mr. Schwartz and Dr. Koppel may be deemed to share voting and dispositive power over the shares held by the Bain Capital Life Sciences Entities. The address of the Bain Capital Life Sciences Entities is c/o Bain Capital Life Sciences, LP, 200 Clarendon Street, Boston, MA 02116.
(2)As reported on a Schedule 13G filed with the SEC on February 2, 2021. Consists of 2,895,054 shares of our common stock reported by BlackRock, Inc. as beneficially owned by the following subsidiaries: BlackRock Advisors, LLC,Blackrock Investment Management (UK) Limited, Blackrock Capital Management, Inc., Blackrock Asset Management Canada Limited, Blackrock (Luxembourg) S.A., Blackrock Fund Advisors, Blackrock Asset Management Ireland Limited, Blackrock Institutional Trust Company, National Association, BlackRock Financial Management, Inc., Blackrock Japan Co., Ltd., Blackrock Asset Management Schweiz AG, and BlackRock Investment Management, LLC. The principal business address of Blackrock, Inc. is 55 East 52nd Street, New York, NY 10055.
(3)As reported on a Schedule 13G/A filed with the SEC on February 8, 2021.9, 2023. Consists of 6,545,1649,139,070 shares of our common stock held by FMR LLC. Abigail P. Johnson is a Director, the Chairman and the Chief Executive Officer of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act, or Fidelity Funds, advised by Fidelity Management & Research Company LLC, or FMR Co. LLC, a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees.
FMR Co. LLC carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. The Schedule 13G filed on February 8, 20219, 2023 by FMR LLC and Abigail P. Johnson reflects the securities beneficially owned, or that may be deemed to be beneficially owned, by FMR LLC, certain of its subsidiaries and affiliates, and other companies, or collectively referred to as the FMR Reporters. Such filing does not reflect securities, if any, beneficially owned by certain other companies whose beneficial ownership of securities is disaggregated from that of the FMR Reporters in accordance with Securities and Exchange Commission Release No. 34-39538 (January 12, 1998).
(4)(2)As reported on a Schedule 13D/A filed with the SEC on FebruaryAugust 9, 2021.2022. Consists of 10,542,790(i) 8,684,232 shares of our common stock held directly by Frazier Life Sciences VIII, LP, or FLS LP. The general partner of FLS LP isL.P. FHM Life Sciences VIII, LP, orL.P. is the general partner of Frazier Life Sciences VIII, L.P. and FHM LP. TheLife Sciences VIII, L.L.C. is the general partner of FHM LP is FHM Life Sciences VIII, LLC.L.P. James N. Topper and Patrick J. Heron are the sole managing members of FHM Life Sciences VIII, LLCL.L.C. and therefore share voting and investment power with respect to suchover the shares held by FLS LP. Dr. TopperFrazier Life Sciences VIII, L.P. and Mr. Heron disclaim beneficial ownership of such shares except to the extent of their pecuniary interest in such shares.FHM Life Sciences VIII, L.L.C. The principal business address of FLS LP is Two Union Square, 601 Union Street, Suite 3200, Seattle, WA 98101.
(3)As reported on a Schedule 13D/13G/A filed with the SEC on February 9, 2021.3, 2023. Consists of (i) 4,267,5645,214,258 shares of our common stock heldreported by OrbiMed Private Investments VII, LP, or OPI VII, (ii) 902,286BlackRock, Inc. as beneficially owned by the following subsidiaries: BlackRock Advisors, LLC, Blackrock Investment Management (UK) Limited, Blackrock Capital Management, Inc., Blackrock Asset Management Canada Limited, Blackrock (Luxembourg) S.A., Blackrock Fund Advisors, Blackrock Asset Management Ireland Limited, Blackrock Institutional Trust Company, National Association, BlackRock Financial Management, Inc., Blackrock Japan Co., Ltd., Blackrock Asset Management Schweiz AG, and BlackRock Investment Management, LLC. The principal business address of Blackrock, Inc. is 55 East 52nd Street, New York, NY 10055.
(4)As reported on a Schedule 13G filed with the SEC on February 8, 2023. State Street Corporation has shares and dispositive voting power over 3,679,385 shares of our common stock held by OrbiMed Partners Master Fund Limited, or OPM, and (iii) 904,000 shares held by Worldwide Healthcare Trust PLC, or WWH. OrbiMed Capital GP VII LLC, or OrbiMed GP VII,stock. The address for this entity is the general partner of OPI VII and OrbiMed Advisors LLC, or OrbiMed Advisors, a registered investment advisor under the Investment Advisors Act of 1940, as amended, is the managing member of OrbiMed GP VII. By virtue of such relationships, OrbiMed GP VII and OrbiMed Advisors may be deemed to have voting and investment power over the securities held by OPI VII and as a result may be deemed to have beneficial ownership over such securities. OrbiMed Capital LLC, or OrbiMed Capital, has the power to direct the vote and disposition of the shares held by OPM and WWH. OrbiMed Capital is a relying adviser of OrbiMed Advisors. OrbiMed Advisors and OrbiMed Capital exercise voting and investment power through a management committee comprised of Carl L. Gordon, Sven H. Borho, and Jonathan T. Silverstein, each of whom disclaims beneficial ownership of the shares held by OPI VII, OPM and WWH. The business address of these entities is c/o OrbiMed Advisors LLC, 601 Lexington Avenue, 54th Floor, New York, NY 10022.State Street Financial Center, One Lincoln Street, Boston, Massachusetts 02111.
(6)(5)Consists of (i) 540,966202,382 shares of our common stock held of record by Todd Franklin Watanabe, (ii) 124,956 shares of our common stock held of record by The Watanabe 2016 Irrevocable Trust, (iii) 49,98157,358 shares of our common stock held of record by Watanabe Ventures, LLC, (iv) 17,850 shares of our common stock held of record by The Anderson Prest Watanabe Irrevocable Trust dated 12 December 2006, (v) 17,850 shares of our common stock held of record by The John Franklin Watanabe Trust dated 25 July 2001, and (vi) 449,656540,421 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021,3, 2023, of which 337,32994,207 are unvested, but early exercisable.
(7)(6)Consists of (i)15,417 8,126 shares of our common stock held of record by Kenneth A. Lock andMasaru Matsuda, (ii) 175,88076,186 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021,3, 2023, and (iii) 4,875 shares of which 122,984 sharesour common stock that are unvested, but early exercisable.subject to vesting of performance units within 60 days of April 3, 2023.
(8)(7)Consists of 1,800(i) 8,299 shares of our common stock held of record by Patrick E. Burnett and (ii) 260,474 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021.3, 2023.
(9)(8)Consists of (i) 201,53625,454 shares of our common stock held of record by Kenneth Lock and (ii) 203,465 shares of our common stock subject to options that are exercisable within 60 days of April 3, 2023, of which 29,512 shares are unvested, but early exercisable.
(9)Consists of (i) 25,740 shares of our common stock held of record by Scott Burrows and (ii) 98,145 shares of our common stock subject to options that are exercisable within 60 days of April 3, 2023.
(10)Consists of (i) 159,263 shares of our common stock held of record by Howard G. Welgus, (ii) 24,991 shares of our common stock held of record by the Welgus Living Trust, UA 02-15-2011, and (iii) 120,744120,201 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021,3, 2023, of which 112,512 shares17,196 are unvested, but early exercisable.exercisable, and (iv) 4,312 shares of our common stock subject to the vesting of restricted stock units within 60 days of April 3, 2023.
(10)(11)Consists of (i) 871,391841,391 shares of our common stock held of record by Bhaskar Chaudhuri, (ii) 49,981 shares of our common stock held of record by the Chaudhuri Family Trust Dated January 12, 2001, and (iii) 57,321149,152 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021.3, 2023, of which 20,827 are unvested, but early exercisable, and (iv) 4,312 shares of our common stock subject to the vesting of restricted stock units within 60 days of April 3, 2023.
(11)(12)Consists of 37,500(i) 50,187 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021,3, 2023, and (ii) 4,312 shares of which all are unvested, but early exercisable.our common stock subject the vesting of restricted stock units within 60 days of April 3, 2023.
(12)(13)Consists of 37,500(i) 62,687 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021,3, 2023, of which 25,00012,500 are unvested, but early exercisable.
(13)Consists of (i) 10,542,790exercisable, and (ii) 4,312 shares of our common stock held by Frazier Life Sciences VIII, LP, or FLS LP, an (ii) 24,000subject to the vesting of restricted stock units within 60 days of April 3, 2023.
(14)Consists of (i) 62,687 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021. The general partner3, 2023, and (ii) 4,312 shares of FLS LP is FHM Life Sciences VIII, LP, or FHM LP. The general partnerour common stock subject the vesting of FHM LP is FHM Life Sciences VIII, LLC. James Topper and Patrick J. Heron are the sole managing membersrestricted stock units within 60 days of FHM Life Sciences VIII, LLC and share voting and investment power with respect to such shares held by FLS LP. Dr. Topper and Mr. Heron disclaim beneficial ownership of such shares except to the extent of their pecuniary interest in such shares. The principal business address of FLS LP is Two Union Square, 601 Union Street, Suite 3200, Seattle, WA 98101.April 3, 2023.
(14)(15)Consists of (i) 6,073,8508,684,232 shares of our common stock held by OrbiMed andFrazier Life Sciences VIII, LP, or FLS LP, (ii) 24,00049,187 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021. Jonathan T. Silverstein is a member of OrbiMed Advisors LLC3, 2023, and a member(iii) 4,312 shares of our boardcommon stock subject to restricted stock units that are vesting within 60 days of directors.April 3, 2023. See footnote 3.(2).
(15)(16)Consists of 24,000(i) 1,750 shares of our common stock held of record by the Leonard Family Trust dated August 28, 1996, (ii) 22,765 shares of our common stock subject to options that are exercisable within 60 days of April 13, 2021. Does not include3, 2023, and (ii) 4,312 shares of our common stock subject the vesting of restricted stock units within 60 days of April 3, 2023.
(17)Consists of (i) 300 shares of our common stock held of record by Sue Jean Lin, (ii) 20,370 shares of our common stock subject to options that are exercisable within 60 days of April 3, 2023, and (ii) 4,312 shares of our common stock subject the Bain Capital Life Sciences Entities (as defined above). See footnote 1. Ricky Sun is a Partner with Bain Capital Life Sciences Investors, LLC.vesting of restricted stock units within 60 days of April 3, 2023.
(16)(18)Consists of 1,232 shares of our common stock held of record by Neha Krishnamohan.
(19)Includes 1,232,759(i) 2,138,587 shares subject to options held by all executive officers and directors that are exercisable within 60 days of April 13, 2021,3, 2023, of which 813,189220,145 shares are unvested, but early exercisable.exercisable, and (ii) 39,371 shares of our common stock subject the vesting of restricted stock units within 60 days of April 3, 2023.
DELINQUENT SECTIONDelinquent Section 16(a) REPORTSReports
Section 16(a) of the Exchange Act requires the Company’sour directors and executive officers, and persons who own more than 10% of a registered class of the Company’sour equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other equity securities of the Company.securities. Officers, directors and greater than 10% stockholders are required by SEC regulations to furnish the Companyus with copies of all Section 16(a) forms they file.
To the Company’sour knowledge, based solely on a review of the copies of such reports furnished to the Companyus and written representations that no other reports were required, the Company believeswe believe that all Section 16(a) filing requirements applicable to our officers, directors and greater than 10% beneficial owners were complied with during the year ended December 31, 2020.2022, except for: (i) due to an administrative oversight, a Form 4/A filed on August 4, 2022 to correct the number of options exercised and (ii) due to an administrative oversight, a Form 4 due on May 29, 2022, on behalf of Mr. Matsuda to report the vesting of 4,875 performance stock units was filed on June 2, 2022.
PROPOSAL NO. 3 APPROVAL, ON A NON-BINDING ADVISORY BASIS, OF THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS
Background
As required by Section 14A(a)(1) of the Exchange Act, the below resolution enables our stockholders to vote to approve, on a non-binding advisory basis, the compensation of our named executive officers as disclosed in this Proxy Statement. This proposal (the “Say-on-Pay Vote”), and commonly known as a “say-on-pay” proposal, gives our stockholders the opportunity to express their views on our named executive officers’ compensation. The Say-on-Pay Vote is not intended to address any specific item of compensation, but rather the overall compensation of our named executive officers and the philosophy, policies and practices described in this Proxy Statement.
We encourage our stockholders to review the “Executive Compensation” section of this Proxy Statement for more information.
As an advisory approval, this proposal is not binding upon us or our Board. However, the Compensation Committee, which is responsible for the design and administration of our executive compensation program, values the opinions of our stockholders expressed through your vote on this proposal. The Board and Compensation Committee will consider the outcome of this vote in making future compensation decisions for our named executive officers. Accordingly, we ask our stockholders to vote “FOR” the following resolution at the Annual Meeting:
“RESOLVED, that the stockholders of Arcutis Biotherapeutics, Inc. approve, on an advisory basis, the fiscal year 2022 compensation of Arcutis Biotherapeutics, Inc.’s named executive officers as described in the “Executive Compensation” section and disclosed in the Summary Compensation Table and related compensation tables and narrative disclosure set forth in Arcutis Biotherapeutics, Inc.’s Proxy Statement for the 2023 Annual Meeting of Stockholders.”
Board Recommendation
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE RESOLUTION TO APPROVE, ON A NON-BINDING ADVISORY BASIS, THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS, AS DISCLOSED IN THE “EXECUTIVE COMPENSATION” SECTION, THE ACCOMPANYING COMPENSATION TABLES AND RELATED NARRATIVE DISCLOSURE OF THIS PROXY STATEMENT.
ADDITIONAL INFORMATION
Householding of Proxy Materials
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for proxy statements and annual reports with respect to two or more stockholders sharing the same address by delivering a single proxy statement addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
Brokers with account holders who are our stockholders may be “householding” our proxy materials. A single proxy statement may be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that it will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you notify your broker or the Company that you no longer wish to participate in “householding.”
If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate proxy statement and annual report, you may (1) notify your broker, (2) direct your written request to: 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361 or (3) request from Broadridge Financial Solutions, Inc. by calling 1-866-540-7095. Stockholders who currently receive multiple copies of this Proxy Statement at their address and would like to request “householding” of their communications should contact their broker. In addition, the Company will promptly deliver, upon written or oral request to the address or telephone number above, a separate copy of the Form 10-K, Proxy Statement, Proxy Card or Notice of Internet Availability of Proxy Materials to a stockholder at a shared address to which a single copy of the documents was delivered.
Forward-Looking Statements
This proxy statement contains “forward-looking statements” (as defined in the Private Securities Litigation Reform Act of 1995). These statements are based on our current expectations and involve risks and uncertainties, which may cause results to differ materially from those set forth in the statements. The forward-looking statements may include statements regarding actions to be taken by us. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Forward-looking statements should be evaluated together with the many uncertainties that affect our business, particularly those mentioned in the risk factors in Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2022 and in our periodic reports on Form 10-Q and our current reports on Form 8-K.
No Incorporation By Reference
In our filings with the SEC, information is sometimes “incorporated by reference.” This means that we are referring you to information that has previously been filed with the SEC, information that should be considered as part of the filing that you are reading. Based on SEC regulations, the reports of the Audit Committee and Compensation Committee included in this proxy statement, are not specifically incorporated by reference into any other filings that we make with the SEC. In addition, references to our website are not intended to function as a hyperlink and the information contained on our website is not intended to be part of this proxy statement. Information on our website, other than our proxy statement, Notice of Annual Meeting of Stockholders, and form of proxy, is not part of the proxy soliciting material and is not incorporated herein by reference.
Other Matters
As of the date of this Proxy Statement, the Board does not intend to present any matters other than those described herein at the Annual Meeting and is unaware of any matters to be presented by other parties. If other matters are properly brought before the Annual Meeting for action by the
stockholders, proxies will be voted in accordance with the recommendation of the Board or, in the absence of such a recommendation, in the discretion of the proxy holder.
We have filed our Annual Report on Form 10-K for the year ended December 31, 20202022 with the SEC. It is available free of charge at the SEC’s web site at www.sec.gov. Upon written request by a stockholder of Arcutis Biotherapeutics, Inc., we will mail without charge a copy of our Annual Report on Form 10-K, including the financial statements and financial statement schedules, but excluding exhibits to the Annual Report on Form 10-K. Exhibits to the Annual Report on Form 10-K are available upon payment of a reasonable fee, which is limited to our expenses in furnishing the requested exhibit. All requests should be directed to the Corporate Secretary, 3027 Townsgate Road, Suite 300, Westlake Village, CA 91361.
| | | | | | | | |
| | By Order of the Board of Directors |
| | |
| | /s/ Todd Franklin Watanabe |
| | Todd Franklin Watanabe |
| | President, Chief Executive Officer and Director |
April 27, 202118, 2023 | | |
SAMPLE